Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
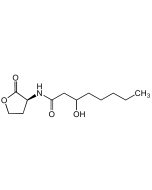
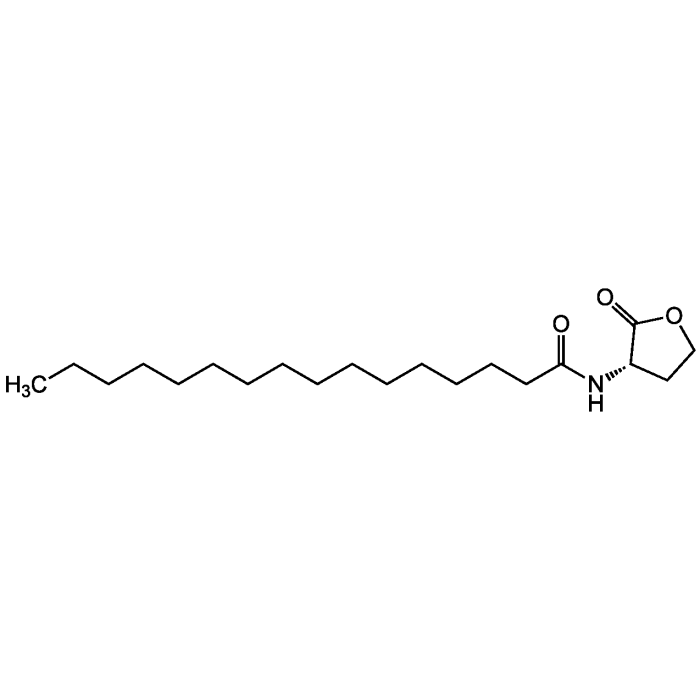
N-Hexadecanoyl-L-homoserine lactone
| Product Details | |
|---|---|
| Synonyms | C16-HSL; N-Palmitoyl-L-homoserine |
| Product Type | Chemical |
| Properties | |
| Formula | C20H37NO3 |
| MW | 339.5 |
| CAS | 87206-01-7 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥97% (GC) |
| Appearance | White solid. |
| Solubility | Soluble in chloroform. Ethanol or methanol are not recommended as solvents as they have been shown to open the lactone ring. |
| Identity | Determined by NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | QJIXVOQAEZMUIH-SFHVURJKSA-N |
| Smiles | CCCCCCCCCCCCCCCC(=O)N[C@H]1CCOC1=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
N-Hexadecanoyl-L-homoserine lactone is a small diffusible signaling molecule and is a member of N-acyl-homoserine lactone family. N-acylhomoserine lactones (AHL) are involved in quorum sensing, controlling gene expression, and cellular metabolism. C16-HSL is a lipophilic, long acyl side-chain bearing AHL, produced by the LuxI AHL synthase homolog SinI involved in quorum-sensing signaling in S. meliloti, a nitrogen-fixing bacterial symbiont of certain legumes. C16-HSL is the most abundant AHL produced by the proteobacterium R. capsulatus and activates genetic exchange between R. capsulatus cells. It tends to localize in relatively lipophilic cellular environments of bacteria and cannot diffuse freely through the cell membrane. The long-chain N-acylhomoserine lactones may be exported from cells by efflux pumps or may be transported between communicating cells by extracellular outer membrane vesicles.
(1) A.L. Schaefer, et al.; J. Bacteriol. 184, 6515 (2002) | (2) M.M. Marketon, et al.; J. Bacteriol. 184, 5686 (2002) | (3) M. Teplitski, et al.; Arch. Microbiol. 180, 494 (2003) | (4) I. Llamas, et al.; Appl. Enviro. Microbiol. 70, 3715 (2004) | (5) M. Gao, et al.; J. Bacteriol. 187, 7931 (2005) | (6) G.D. Geske, et al.; ChemBioChem 9, 389 (2008)