Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
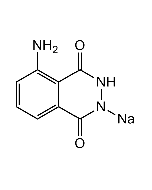
Luminol
| Product Details | |
|---|---|
| Synonyms | 3-Aminophthalhydrazide; 5-Amino-2,3-dihydro-1,4-phthalazinedione; NSC5064 |
| Product Type | Chemical |
| Properties | |
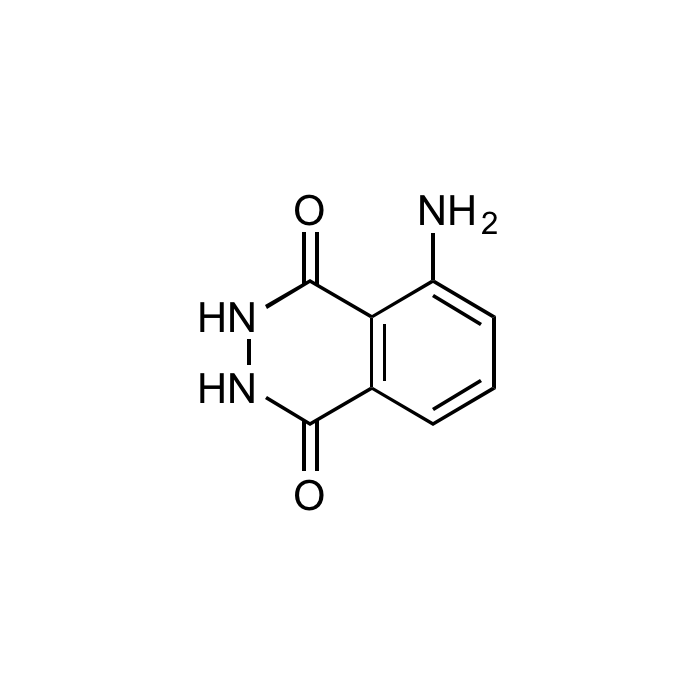
| Formula | C8H7N3O2 |
| MW | 177.16 |
| CAS | 521-31-3 |
| RTECS | TH8890060 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥97% (Titration) |
| Appearance | White to light yellow powder. |
| Solubility | Soluble in DMSO (50mg/ml). Insoluble in water. |
| Identity | Determined by NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | HWYHZTIRURJOHG-UHFFFAOYSA-N |
| Smiles | NC1=CC=CC2=C1C(=O)NNC2=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Very efficient and commonly used chemiluminiscent compound in many applications. Cyclic diacyl hydrazide that exhibits chemiluminescence upon oxidation. Oxidizing agents capable of initiating this particular reaction are nitrogen dioxide, PAN, hydrogen peroxide, ozone and other atmospheric oxidants. As an example, HRP combined with hydrogen peroxide as an activator, causes luminescent peroxidation of luminol. This reaction can be enhanced by certain phenol derivatives, such as p-substituted phenols. Luminol can also be oxidized and chemiluminesce, by compounds containing iron, copper, gold, and cyanide. Luminol is a reagent for the generation of chemiluminescence in biological assays including nitric oxide and myeloperoxidase assays or in fornensic blood tests. Spectral data: λex=355nm, λem=411nm.
(1) M.J. Cormier & P.M. Prichard; J. Biol. Chem. 243, 4706 (1968) | (2) G.H.G. Thorpe, et al.; Clin. Chem. 31, 1335 (1985) | (3) T.I. Quickenden & J.I. Creamer; Luminescence 16, 295 (2001) | (4) A. Fan, et al.; Anal. Sci. 25, 5877 (2009) | (5) Y.A. Vladimirov, et al.; Biochem. 74, 1545 (2009)






![4-[4'-(2'-Methyl)thiazolyl]phenol](https://adipogen.com/media/catalog/product/cache/60eb5af712bc93baae8d55513bd31b01/i/m/image_315.png)