Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
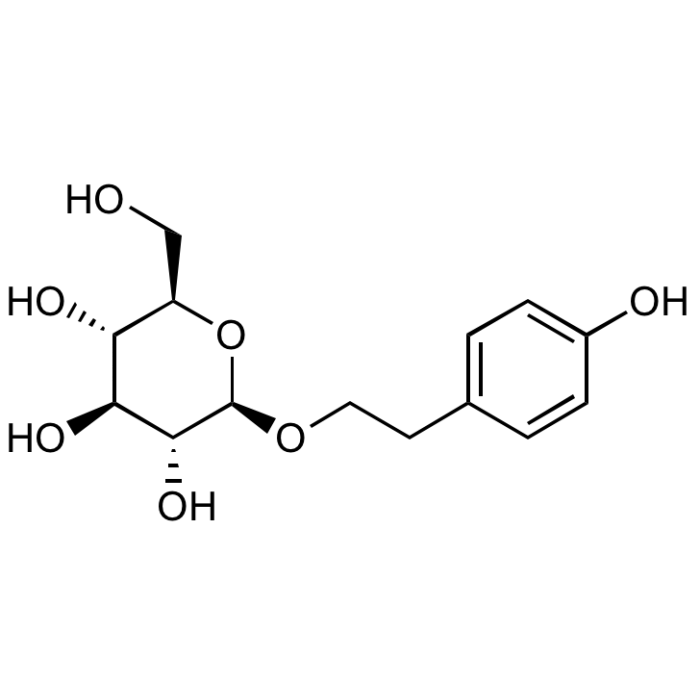
Salidroside
| Product Details | |
|---|---|
| Synonyms | Rhodioloside; 2-(4-Hydroxyphenyl)ethyl β-D-glucopyranoside; p-Hydroxyphenethyl glucopyranoside |
| Product Type | Chemical |
| Properties | |
| Formula | C14H20O7 |
| MW | 300.30 |
| CAS | 10338-51-9 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO (60mg/ml), water (60mg/ml) or ethanol (4mg/ml). |
| Identity | Determined by NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | ILRCGYURZSFMEG-RKQHYHRCSA-N |
| Smiles | OC[C@H]1O[C@@H](OCCC2=CC=C(O)C=C2)[C@H](O)[C@@H](O)[C@@H]1O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Glycoside isolated from plants, most commonly from members of the genus Rhodiola, with many biological activities. Shown to have neuroprotective, antioxidant, antiviral, antidepressant, cardioprotective, anti-inflammatory and anticancer activity. Ameliorates insulin resistance.
(1) P. Yu, et al.; Chem. Biodivers. 4, 508 (2007) | (2) H. Wang, et al.; Phytomedicine 16, 146 (2009) | (3) X. Hu, et al.; BBRC 398, 62 (2010) | (4) L. Zhang, et al.; Neurochem. Int. 57, 547 (2010) | (5) Y. Guo, et al.; Chem. Pharm. Bull. 58, 1327 (2010) | (6) Y. Tang, et al.; Br. J. Pharmacol. 171, 2440 (2014) | (7) S.J. Yang, et al.; Pharmacol. Biochem. Behav. 124, 451 (2014) | (8) T. Zheng, et al.; Br. J. Pharmacol. 172, 3284 (2015) | (9) G. Zhao, et al.; Oncol. Rep. 33, 2553 (2015) | (10) S.S. Xing, et al.; Vascul. Pharmacol. 72, 141 (2015) | (11) L. Zhu, et al.; Apoptosis 20, 1433 (2015)