Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
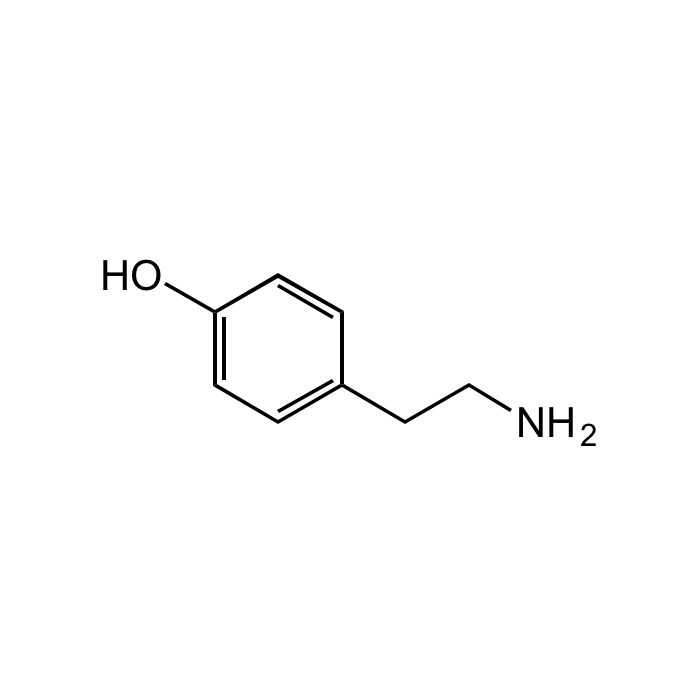
Tyramine
| Product Details | |
|---|---|
| Synonyms | 2-(4-Hydroxyphenyl)ethylamine; 4-(2-Aminoethyl)phenol; 4-Hydroxyphenethylamine; NSC 249188; p-Tyramine; Uteramine |
| Product Type | Chemical |
| Properties | |
| Formula |
C8H11NO |
| MW | 137.2 |
| CAS | 51-67-2 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥98% (NMR) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO, methanol or ethanol. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | DZGWFCGJZKJUFP-UHFFFAOYSA-N |
| Smiles | OC1=CC=C(CCN)C=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Tyramine is a naturally-occurring trace amine derived from the amino acid tyrosine that is produced endogenously or can be obtained from certain foods. Tyramine acts as a catecholamine releasing agent. It is unable to cross the blood-brain barrier, resulting in only non-psychoactive peripheral sympathomimetic effects following ingestion. Acts as a adrenergic and activates trace amine-associated receptor 1. Tyramine also inhibits the release of norepinephrine and dopamine. It is metabolized by monoamine oxidase A. Primarily used as an intermediate in the manufacture of pharmaceutical and agrochemical products.
(1) A.J. Gross & I.W. Sizer; J. Biol. Chem.. 234, 1611 (1959) | (2) F. Sjoeqvist; Proc. R. Soc. Med. 58, 967 (1965) | (3) M. Da Prada, et al.; J. Neural. Transm. Suppl. 26, 31 (1988) | (4) R.B. Rothman, et al.; Science 39, 32 (2001) | (5) R. Zucchi, et al.; Br. J. Pharmacol. 149, 967 (2006) | (6) E.A. Reese, et al.; J. Pharmacol. Exp. Therap. 321, 178 (2007) | (7) J.J. Maguire, et al.; Pharmacol. Rev. 61,1 (2009)