Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
SynKinase
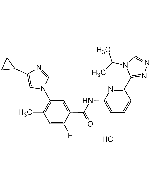
Selonsertib (free base)
As low as
CHF 0.00
In stock
Only %1 left
SYN-1231-M0011 mgCHF 128.00
SYN-1231-M0055 mgCHF 270.00
SYN-1231-M01010 mgCHF 412.00
SYN-1231-M05050 mgCHF 1’263.00
SYN-1231-M100100 mgINQ
| Product Details | |
|---|---|
| Synonyms | GS-4997 (free base); 5-(4-Cyclopropyl-1H-imidazol-1-yl)-2-fluoro-N-(6-(4-isopropyl-4H-1,2,4-triazol-3-yl)pyridin-2-yl)-4-methylbenzamide |
| Product Type | Chemical |
| Properties | |
| Formula | C24H24FN7O |
| MW | 445.5 |
| CAS | 1448428-04-3 |
| Purity Chemicals | ≥95% |
| Appearance | Solid. |
| Solubility | Soluble in DMSO. Insoluble in water. |
| Declaration | Manufactured by SynKinase. |
| Other Product Data |
Target: ASK1 | Kinase Group: Ser/Thr Kinases | Click here for Original Manufacturer Product Datasheet Our product description may differ slightly from the original manufacturers product datasheet. |
| InChi Key | YIDDLAAKOYYGJG-UHFFFAOYSA-N |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Keep cool and dry. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
Selonsertib, also known as GS-4997, is an orally bioavailable inhibitor of apoptosis signal-regulating kinase 1 (ASK1), with potential anti-inflammatory, antineoplastic and anti-fibrotic activities. GS-4997 targets and binds to the catalytic kinase domain of ASK1 in an ATP-competitive manner. GS-4997 prevents the production of inflammatory cytokines, down-regulates the expression of genes involved in fibrosis, suppresses excessive apoptosis and inhibits cellular proliferation.
Product References
- Design of a phase 2 clinical trial of an ASK1 inhibitor, GS-4997, in patients with diabetic kidney disease: J.H. Lin, et al.; Nephron 129, 29 (2015)
- A Quantitative Framework to Evaluate Proarrhythmic Risk in a First-in-Human Study to Support Waiver of a Thorough QT Study: C.H. Nelson, et al.; Clin. Pharmacol. Ther. 98, 630 (2015)