Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
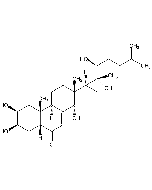
Muristerone A
As low as
75
CHF
CHF 75.00
In stock
Only %1 left
AG-CN2-0070-C250250 µgCHF 75.00
AG-CN2-0070-M0011 mgCHF 190.00

| Product Details | |
|---|---|
| Synonyms | 2β,3β,5β,11α,14α,20R,22R-Heptahydroxycholest-7-en-6-one |
| Product Type | Chemical |
| Properties | |
| Formula |
C27H44O8 |
| MW | 496.6 |
| CAS | 38778-30-2 |
| Source/Host Chemicals | Isolated from Ipomoea hederacea seeds. |
| Purity Chemicals | ≥95% (HPLC) |
| Appearance | White to off-white solid. |
| Solubility | Soluble in acetic acid, ethanol, methanol or DMSO. Insoluble in water. |
| Identity | Identity determined by 1H-NMR. |
| Other Product Data |
To prepare a 1mM stock solution (1.1mM) in 100% ethanol: Add 2ml of 100% ethanol to 1mg muristerone A (2µmol). Gently mix to dissolve the product (5 to 10min.). Do not heat solution to dissolve the product. Store the 1mM stock solution at +4°C or at -20°C. |
| InChi Key | LRJUYAVTHIEHAI-LDJCFVSYSA-N |
| Smiles | [H][C@@]1(CC[C@@]2(O)C3=CC(=O)[C@]4(O)C[C@@H](O)[C@@H](O)C[C@]4(C)C3[C@H](O)C[C@]12C)[C@@](C)(O)[C@H](O)CCC(C)C |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Potent member of the ecdysteroid family.
- Ecdysone receptor (EcR) agonist.
- Analog of ecdysone with similar properties to ponasterone A (Prod. No. AG-CN2-0053).
- Inducer of ecdysone-inducible gene expression systems in mammalian cells and transgenic animals.
- Induces apoptosis in cells transfected with wild-type Bax.
- Induces expression of β-galactosidase.
- Stimulates Bcl-XL mRNA transcription and inhibits TRAIL- and hFasL-induced apoptosis in RKO cells.
- Insect steroid hormone involved in regulating metamorphosis, causing a response to G2 cell cycle arrest.
- Major molting hormone in some insects. Has protective effects in plants.
Product References
- Structure of muristerone A, a new phytoecdysone: L. Canonica, et al.; J. C. S. Chem. Commun. 1060 (1972)
- New phytoecdysones from kaladana. I. Structure of muristerone A and kaladasterone: L. Canonica, et al.; Gazz. Chim. Ital. 107, 123 (1977)
- Characterization and partial purification of the Drosophila Kc cell ecdysteroid receptor: T.M. Landon, et al.; J. Biol. Chem. 263, 4693 (1988)
- Ecdysteroid-Dependent Regulation of Genes in Mammalian Cells by a Drosophila Ecdysone Receptor and Chimeric Transactivators: K. Christopherson, et al.; PNAS 89, 6314 (1992)
- Ecdysone-inducible gene expression in mammalian cells and transgenic mice: D. No, et al.; PNAS 93, 3346 (1996)
- The overexpression of Bax produces cell death upon induction of the mitochondrial permeability transition: J.G. Pastorino, et al.; J. Biol. Chem. 273, 7770 (1998)
- Impairment of the proapoptotic activity of Bax by missense mutations found in gastrointestinal cancers: J. Gil, et al.; Cancer Res. 59, 2034 (1999)
- Identification of ligands and coligands for the ecdysone-regulated gene switch: E. Saez, et al.; PNAS 97, 14512 (2000)
- Ectopic expression of cyclin D1 impairs the proliferation and enhances the apoptosis of a murine lymphoid cell line: F. Duquesne, et al.; Cell Death Differ. 8, 51 (2001)
- The ecdysone inducible gene expression system: unexpected effects of muristerone A and ponasterone A on cytokine signaling in mammalian cells: S. Constantino, et al.; Eur. Cytokine Netw. 12, 365 (2001)
- Muristerone A-induced nerve growth factor release from genetically engineered human dermal fibroblasts for peripheral nerve tissue engineering: C.W. Patrick, et al.; Tissue Eng. 7, 303 (2001)
- Agonists of an ecdysone-inducible mammalian expression system inhibit Fas-ligand- and TRAIL-induced apoptosis in the human colon carcinoma cell line RKO: I Oehme, et al.; Cell Death Diff. 13, 189 (2005)
- Selected technologies to control genes and their products for experimental and clinical purposes: H.K. Alexander, et al.; Arch. Immunol. Ther. Exp. 55, 139 (2007) (Review)