Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
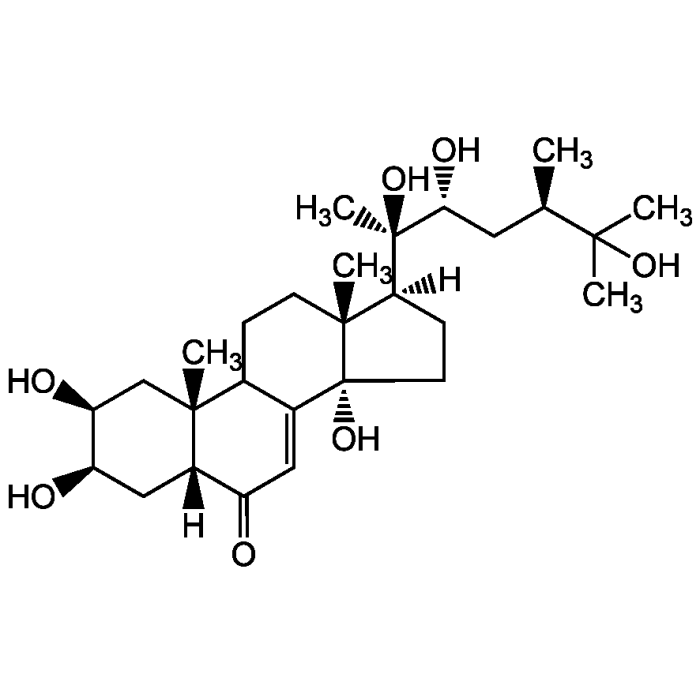
Makisterone A
As low as
55
CHF
CHF 55.00
In stock
Only %1 left
AG-CN2-0073-C250250 µgCHF 55.00
AG-CN2-0073-M0011 mgCHF 150.00
AG-CN2-0073-M0055 mgCHF 450.00
| Product Details | |
|---|---|
| Synonyms | 2β,3β,14α,20R,22R,25-Hexahydroxy-5β-24R-ergost-7-en-6-one |
| Product Type | Chemical |
| Properties | |
| Formula |
C28H46O7 |
| MW | 494.7 |
| CAS | 20137-14-8 |
| Source/Host Chemicals | Isolated from Ipomoea hederacea. |
| Purity Chemicals | ≥95% (HPLC) |
| Appearance | White to off-white solid. |
| Solubility | Soluble in acetic acid, ethanol, methanol or DMSO. Sparingly soluble in chloroform. Insoluble in water. |
| Identity | Identity determined by 1H-NMR. |
| InChi Key | IJRBORPEVKCEQD-WJUVRXFPSA-N |
| Smiles | [H][C@@]1(CC[C@@]2(O)C3=CC(=O)[C@]4([H])C[C@@H](O)[C@@H](O)C[C@]4(C)C3CC[C@]12C)[C@@](C)(O)[C@H](O)C[C@@H](C)C(C)(C)O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- A member of the ecdysteroid family.
- Ecdysone receptor (EcR) agonist.
- Induces the expression of genes coding for proteins that the larva requires, and it causes chromosome puffs (sites of high expression) to form in polytene chromosomes.
- Plays a role in insect development, cell proliferaton, growth and apoptosis by controlling gene expression involved in moulting and metamorphosis. It acts through a heterodimeric receptor comprising the ecdysone receptor and the ultraspiracle proteins (USP).
- Appears in plants mostly as a protection agent (toxins or antifeedants) against herbivorous insects.
- Could be used for controlled gene expression in scientific research, agriculture and medicine.
- Could be used for the development of selective insect growth regulators for use as environmentally benign insecticides.
Product References
- Paper chromatographic separation of alpha-ecdysone, ecdysterone, inokosterone, makisterone A and ponasterone A: M.W. Gilgan & T.E. Farquharson; Steroids 22, 365 (1973)
- The determination of absolute configuration at C-24 of the phytoecdysone makisterone A: B. Danieli, et al.; J. C. S. Chem. Commun. 745 (1974)
- Makisterone A: a 28-carbon hexahydroxy molting hormone from the embryo of the milkweed bug: J.N. Kaplanis, et al.; Science 190, 681 (1975)
- Evidence for the presence of makisterone A in Drosophila larvae and the secretion of 20-deoxymakisterone A by the ring gland: C.P. Redfern; PNAS 81, 5643 (1984)
- Ecdysteroids increase the yield of recombinant protein produced in baculovirus insect cell expression system: M. Sarvari, et al.; BBRC 167, 1154 (1990)
- The effects of several ecdysteroids and ecdysteroid agonists on two Drosophila imaginal disc cell lines: D.M. Cottam & M.J. Milner; Cell Mol. Life Sci. 53, 600 (1997)
- Ecdysone receptors and their biological actions: L.M. Riddiford, et al.; Vitam. Horm. 60, 1 (2000)
- Ecdysteroids of quinoa seeds (Chenopodium quinoa Willd.): N. Zhu, et al.; J. Agric. Food Chem. 49, 2576 (2001)
- DNA synthesis in the imaginal wing discs of the American bollworm Helicoverpa armigera (Hübner): A. Josephrajkumar & B. Subrahmanyam; J. Biosci. 27, 113 (2002)
- Ecdysone-regulated puff genes 2000: C.S. Thummel; Insect Biochem. Mol. Biol. 32, 113 (2002)
- Ecdysone-controlled expression of transgenes: L.D. Graham; Expert Opin. Biol. Ther. 2, 525 (2002)
- Non-genomic ecdysone effects and the invertebrate nuclear steroid hormone receptor EcR-new role for an "old" receptor? U. Schlattner, et al.; Mol. Cell Endocrinol. 247, 64 (2006)