Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
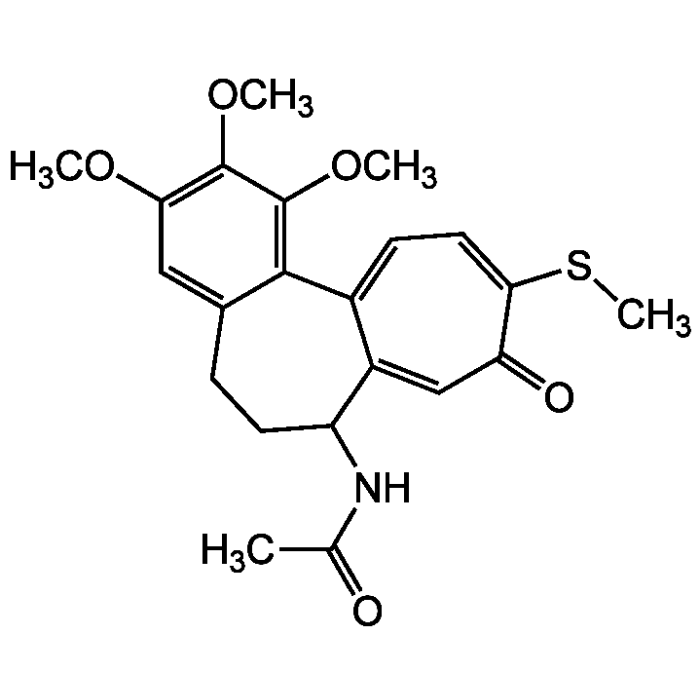
Thiocolchicine
As low as
45
CHF
CHF 45.00
In stock
Only %1 left
AG-CN2-0074-M0055 mgCHF 45.00
AG-CN2-0074-M02525 mgCHF 180.00
| Product Details | |
|---|---|
| Synonyms | 10-Demethoxy-10-methylthiocolchicine; NSC 186301 |
| Product Type | Chemical |
| Properties | |
| Formula |
C22H25NO5S |
| MW | 415.5 |
| CAS | 2730-71-4 |
| RTECS | GH0878800 |
| Source/Host Chemicals | Semisynthetic. |
| Purity Chemicals | ≥95% (NMR) |
| Appearance | Yellow solid. |
| Solubility | Soluble in ethanol or acetone. Almost insoluble in water or ether. |
| Identity | Determined by 1H-NMR. |
| InChi Key | CMEGANPVAXDBPL-UHFFFAOYSA-N |
| Smiles | COC1=C(OC)C(OC)=C2C(CCC(NC(C)=O)C3=CC(=O)C(SC)=CC=C23)=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Antimitotic alkaloid.
- Tubulin polymerization and microtubule assembly inhibitor.
- Axonal cytoskeleton modulator.
- Inhibitor of autophagosome-lysosome fusion.
- Apoptosis inducer.
- Thiocolchicine-dimers were shown to be potent topoisomerase I inhibitors.
Product References
- Association of thiocolchicine with tubulin: R.M. Chabin & S.B. Hastie; BBRC 161, 544 (1989)
- Effect of tubulin binding and self-association on the near-ultraviolet circular dichroic spectra of colchicine and analogues; R.M. Chabin, et al.; Biochemistry 29, 1869 (1990)
- N-acetylcolchinol O-methyl ether and thiocolchicine, potent analogs of colchicine modified in the C ring. Evaluation of the mechanistic basis for their enhanced biological properties: G.J. Kang, et al.; J. Biol. Chem. 265, 10255 (1990)
- Antitumor agents-CLXXV. Anti-tubulin action of (+)-thiocolchicine prepared by partial synthesis: Q. Shi, et al.; Bioorg. Med. Chem. 5, 2277 (1997)
- Antiproliferative activity of colchicine analogues on MDR-positive and MDR-negative human cancer cell lines: R. De Vincenzo, et al.; Anticancer Drug Des. 13, 19 (1998)
- Biological evaluation on different human cancer cell lines of novel colchicine analogs: R. De Vincenzo, et al.; Oncol. Res. 11, 145 (1999)
- Effects of thiocolchicine on axonal cytoskeleton of the rat peroneus nerve: P. Ferri, et al.; Exp. Toxicol. Pathol. 54, 211 (2002)
- Thiocolchicine dimers: a novel class of topoisomerase-I inhibitors: G. Raspaglio, et al.; Biochem. Pharmacol. 69, 113 (2005)
- Inhibitors of tubulin polymerization: synthesis and biological evaluation of hybrids of vindoline, anhydrovinblastine and vinorelbine with thiocolchicine, podophyllotoxin and baccatin III: D. Passarella, et al.; Bioorg. Med. Chem. 16, 6269 (2008)
- Synthesis and biological evaluation of novel thiocolchicine-podophyllotoxin conjugates: D. Passarella, et al.; Eur. J. Med. Chem. 45, 219 (2010)