Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
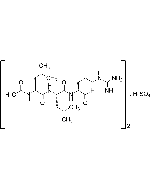
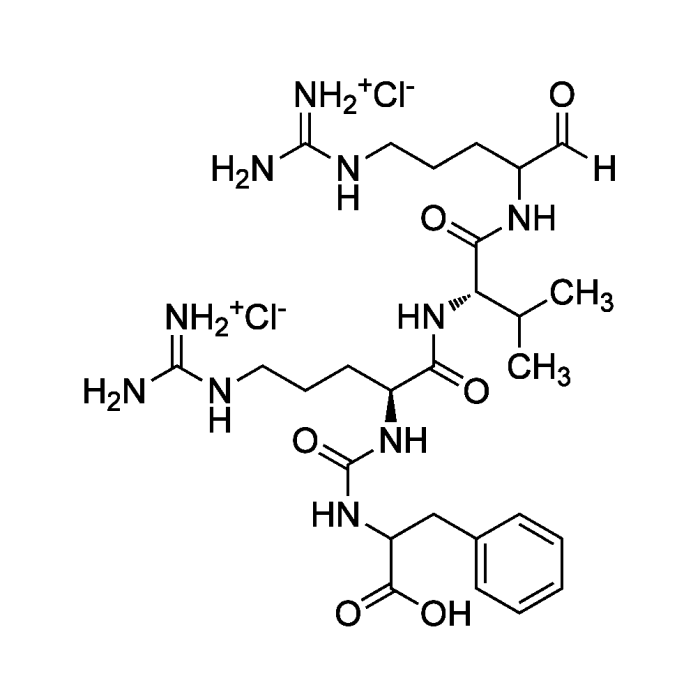
Antipain . dihydrochloride
As low as
45
CHF
CHF 45.00
In stock
Only %1 left
AG-CP3-7002-M0055 mgCHF 45.00
AG-CP3-7002-M02525 mgCHF 170.00
| Product Details | |
|---|---|
| Synonyms | [(S)-1-Carboxy-2-phenylethyl]-carbamoyl-Arg-Val-arginal . 2HCl |
| Product Type | Chemical |
| Properties | |
| Formula |
C27H44N10O6 . 2HCl |
| MW | 604.7 . 72.9 |
| CAS | 37682-72-7 |
| RTECS | YV9350800 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥97% (HPLC) |
| Appearance | White to off-white solid. |
| Solubility | Soluble in water, ethanol, methanol, DMSO or acetonitrile. |
| Biological Activity |
Inhibitory Activity: ≥30 U/mg (Trypsin, L-BAPA) |
| InChi Key | YAHXZYICKJUJEO-BXLPLHKWSA-N |
| Smiles | [Cl-].[Cl-].[H]C(=O)C(CCCNC(N)=[NH2+])NC(=O)[C@@H](NC(=O)[C@H](CCCNC(N)=[NH2+])NC(=O)NC(CC1=CC=CC=C1)C(O)=O)C(C)C |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 3 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Potent reversible inhibitor of serine/cysteine proteases and some trypsin-like proteases. Inhibits papain, trypsin and trypsin-like serine proteases as well as cathepsin A, B and D.
- Similar activity spectrum comparable to leupeptin (AG-CP3-7000).
- Acts by forming a hemiacetal adduct between its aldehyde group and the active serine of the proteinase.
- Inhibits transformation of NIH3T3 cells after transfection with an activated H-ras oncogene.
- Used to evaluate the role of proteases in the process of malignant cell transformations.
- Shows antibacterial and antiparasitic activity.
Product References
- Antipain, a new protease inhibitor isolated from actinomycetes: H. Suda, et al.; J. Antibiot. 25, 263 (1972)
- Structures and activities of protease inhibitors of microbial origin: H. Umezawa; Methods Enzymol. 45, 678 (1976)
- Inhibition of proteolytic activity of calcium activated neutral protease by leupeptin and antipain: T. Toyo-Oka, et al.; BBRC 82, 484 (1978)
- Effects of antipain (a protease inhibitor) on respiration, viability, and excision of pyrimidine dimers in UV-irradiated Escherichia coli cells: P.A. Swenson & R.L. Schenley; J. Bacteriol. 135, 1167 (1978)
- Effect of Ca2+ on the inhibition of calcium-activated neutral protease by leupeptin, antipain and epoxysuccinate derivatives: K. Suzuki, et al.; FEBS Lett. 136, 119 (1981)
- Inhibition of Leishmania amastigote growth by antipain and leupeptin: G.H. Coombs & J. Baxter; Ann. Trop. Med. Parasitol. 78, 21 (1984)
- Antipain or leupeptin in combination with aspirin or indomethacin synergistically inhibit human platelet activation by thrombin and trypsin: M. Ruggiero & E.G. Lapetina; Adv. Prostaglandin Thromboxane Leukot. Res. 17A, 563 (1987)
- Inhibition of H-ras oncogene transformation of NIH3T3 cells by protease inhibitors: S.J. Garte, et al.; Cancer Res. 47, 3159 (1987)
- Reversible conversion of tyrosine hydroxylase to an inactive form by antipain: S. Okuno & H. Fujisawa; BBRC 146, 1375 (1987)
- Antipain-induced suppression of oncogene expression in H-ras-transformed NIH3T3 cells: L.R. Cox, et al.; Cancer Res. 51, 4810 (1991)
- Effects of the protease inhibitor antipain on cell malignant transformation: M. Vaccari, et al.; Anticancer Res. 19, 589 (1999)
- Lysosomes, growth factor activity, and carcinogenic implications: M. Melzer; Crit. Rev. Eukaryot. Gene. Expr. 22, 345 (2012)