Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
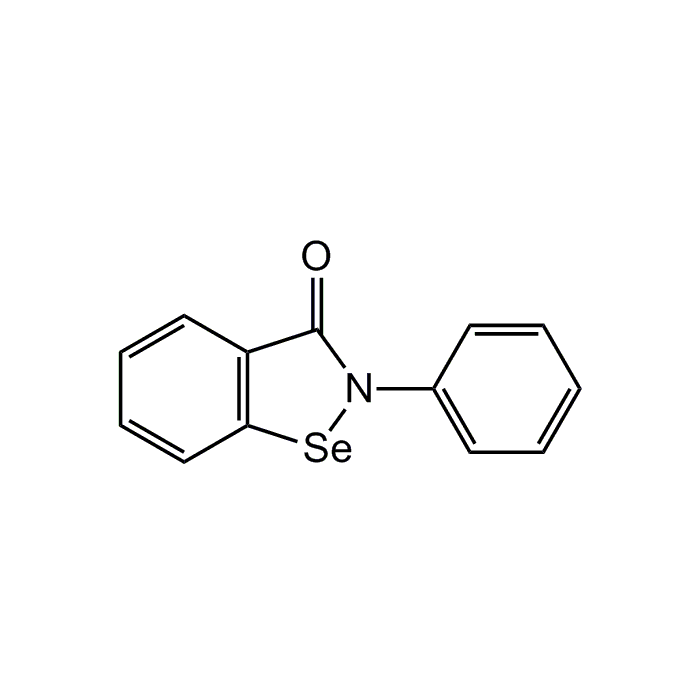
Ebselen
As low as
25
CHF
CHF 25.00
In stock
Only %1 left
AG-CR1-0031-M0011 mgCHF 25.00
AG-CR1-0031-M0055 mgCHF 35.00
AG-CR1-0031-M02525 mgCHF 95.00
| Product Details | |
|---|---|
| Synonyms | 2-Phenyl-1,2-benzisoselenazol-3-(2H)-one |
| Product Type | Chemical |
| Properties | |
| Formula |
C13H9NOSe |
| MW | 274.2 |
| Merck Index | 14: 3486 |
| CAS | 60940-34-3 |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white solid. |
| Solubility | Soluble in DMSO (10mg/ml) or 100% ethanol (5mg/ml). |
| Identity | Determined by 1H-NMR. |
| InChi Key | DYEFUKCXAQOFHX-UHFFFAOYSA-N |
| Smiles | O=C1N([Se]C2=C1C=CC=C2)C1=CC=CC=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Glutathione peroxidase mimetic [1, 4].
- Peroxynitrite scavenger [4].
- Anti-inflammatory [1, 5-7]
- Antioxidant [2, 3, 5].
- Protein kinase C (PKC), NADPH, lipoxygenase, COX, NOS, H+-ATPase and NADPH oxidase inhibitor [6-9].
- Antifungal [9].
- Review [10].
- Potent IDO-1 inhibitor with Ki 94nM [11].
- Inhibitor of HIV-1 capsid C-terminal domain dimerization.
- Shown to potentially inhibit the 33.8-kDa Main Protease (Mpro)/3C-like Protease of SARS-CoV-2, consequently inhibiting viral transcription and replication and possibly inhibiting spread of COVID-19.
Product References
- A novel biologically active seleno-organic compound--III. Effects of PZ 51 (Ebselen) on glutathione peroxidase and secretory activities of mouse macrophages: M.J. Parnham & S. Kindt; Biochem. Pharmacol. 33, 3247 (1984)
- Seleno-organic compounds and the therapy of hydroperoxide-linked pathological conditions: M.J. Parnham & E. Graf; Biochem. Pharmacol. 36, 3095 (1987)
- Kinetic mechanism and substrate specificity of glutathione peroxidase activity of ebselen (PZ51): M. Maiorino, et al.; Biochem. Pharmacol. 37, 2267 (1988)
- Ebselen as a glutathione peroxidase mimic and as a scavenger of peroxynitrite: H. Sies & H. Masumoto; Adv. Pharmacol.38, 229 (1997)
- Ebselen, a glutathione peroxidase mimetic seleno-organic compound, as a multifunctional antioxidant. Implication for inflammation-associated carcinogenesis: Y. Nakamura, et al.; J. Biol. Chem. 277, 2687 (2002)
- Molecular actions of ebselen-an antiinflammatory antioxidant: T. Schewe; Gen. Pharmacol. 26, 1153 (1995)
- Studies on the anti-inflammatory activity of ebselen. Ebselen interferes with granulocyte oxidative burst by dual inhibition of NADPH oxidase and protein kinase C?: I.A. Cotgreave, et al.; Biochem. Pharmacol. 38, 649 (1989)
- Strong inhibition of mammalian lipoxygenases by the antiinflammatory seleno-organic compound ebselen in the absence of glutathione: C. Schewe, et al.; Biochem. Pharmacol. 48, 65 (1994)
- Evaluation of the antifungal and plasma membrane H+-ATPase inhibitory action of ebselen and two ebselen analogs in S. cerevisiae cultures: B. Billack, et al.; J. Enzyme Inhib. Med. Chem. 25, 312 (2010)
- Ebselen: a thioredoxin reductase-dependent catalyst for alpha-tocopherol quinone reduction: J. Fang, et al.; Toxicol. Appl. Pharmacol. 207, 103 (2005)
- The selenazal drug ebselen potently inhibits indoleamine 2,3-dioxygenase by targeting enzyme cysteine residues: A.C. Terentis, et al. Biochem. 49, 591 (2010)
- Repurposing ebselen for treatment of multidrug-resistant staphylococcal infections: S. Thangamani, et al.; Nature Sci. Rep. 5, 11596 (2015)
- Ebselen, a small-molecule capsid inhibitor of HIV-1 replication: S. Thenin-Houssier, et al.; Antimicrob. Agents Chemother. 60, 2195 (2016)
- Screening a Repurposing Library for Inhibitors of Multidrug-Resistant Candida auris Identifies Ebselen as a Repositionable Candidate for Antifungal Drug Development: G. Wall, et al.; Antimicrob. Agents Chemother. 62, e01084 (2018)
- Structure of Mpro from COVID-19 virus and discovery of its inhibitors: Z. Jin, et al.; Nature 582, 289 (2020)
- A Small Molecule, ACAi-028, with Anti-HIV-1 Activity Targets a Novel Hydrophobic Pocket on HIV-1 Capsid: Antimicrob. Agents Chemother. 65, e01039 (2021)