Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
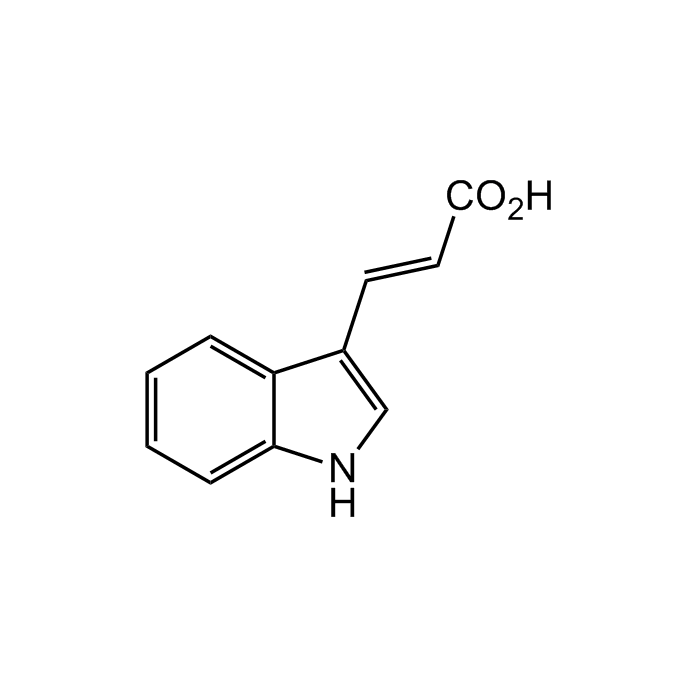
trans-Indole-3-acrylic acid
As low as
40
CHF
CHF 40.00
In stock
Only %1 left
AG-CR1-3677-M250250 mgCHF 40.00
AG-CR1-3677-G0011 gCHF 85.00
| Product Details | |
|---|---|
| Synonyms | IAcrA; trans-3-Indoleacrylic acid; trans-β-Indoleacrylic acid; (E)-3-(1H-Indol-3-yl)acrylic acid; NSC29428; NSC137806; BRN0006317 |
| Product Type | Chemical |
| Properties | |
| Formula |
C11H9NO2 |
| MW | 187.2 |
| CAS | 29953-71-7 |
| Purity Chemicals | ≥98% |
| Appearance | Off-white solid. |
| Solubility | Soluble in DMSO or ethanol. Slightly soluble in water. |
| Other Product Data |
Note: Warming and sonication may be required when dissolving the compound in the solvent of choice. |
| InChi Key | PLVPPLCLBIEYEA-AATRIKPKSA-N |
| Smiles | O=C(/C=C/C1=CNC2=CC=CC=C21)O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Inflammation suppressor. Tryptophan metabolite produced by human microbiota (intestinal commensale Peptostreptococcus sp.). Involved in keeping the intestinal barrier intact and works as anti-inflammatory molecule. Patients with Inflammatory Bowel Disease (IBD) have an altered microbiota, with less Peptostreptococcus sp., and therefore reduced production of trans-indole-3-acrylic acid.
- Chromophoric L-Trp analog used to probe the allosteric properties of the internal aldimine of tryptophan synthase. Also shown to be a moderate inhibitor of tryptophan synthase, trypothan-2,3-dioxygenase (TDO), indoleamine-2,3-dioxygenase (IDO), L-dopachrome isomerase and xanthine oxidase.
- Reagent used as a matrix for MALDI-TOF mass spectroscopy in order to characterize and analyze polyphenols and synthetic polymers.
- Used as heterocyclic building block or intermediate for the synthesis of indolyl acrylamide-derived inhibitors, pharmaceuticals or agrochemicals.
Product References
- Inhibition of tryptophan synthetase by indoleacrylic acid: W.H. Matchett; J. Bacteriol. 110, 146 (1972)
- Stereospecificity of hepatic L-tryptophan 2,3-dioxygenase: Y. Watanabe, et al.; Biochem. J. 189, 393 (1980)
- Inhibition of indoleamine 2,3-dioxygenase and tryptophan 2,3-dioxygenase by beta-carboline and indole derivatives: N. Eguchi; Arch. Biochem. Biophys. 232, 602 (1984)
- The structural basis for the interaction between L-tryptophan and the Escherichia coli trp aporepressor: R.Q. Marmorstein, et al.; J. Biol. Chem. 262, 4922 (1987)
- Interaction of Indoleacrylic Acid with Trp aporepressor from Escherichia coli: D. Hu & M.R. Eftink; Arch. Biochem. Biophys. 305, 588 (1993)
- Photosensizied inactivation of infectious DNA by urocanic acid, indoleacrylic acid and rhodiuk complexes: T. Mohammad, et al.; Photochem. Photobiol. 59, 189 (1994)
- Desorption behavior and distributions of fluorinated polymers in MALDI and electrospray ionization mass spectrometry: L. Latourte, et al.; Anal. Chem. 69, 2742 (1997)
- Substrate specificity for isomerase activity of macrophage migration inhibitory factor and its inhibition by indole derivatives: M. Suzuki, et al.; J. Biochem. 122, 1040 (1997)
- Where does indolylacrylic acid come from?: E. Marklova; Amino Acids 17, 401 (1999)
- Novel allosteric effectors of the tryptophan synthase K2L2 complex identifed by computer-assisted molecular modelling: A. Marabotti, et al.; Biochim. Biophys. Acta 1476, 287 (2000)
- MALDI-TOF mass spectrometry of oligomeric food polyphenols: J.D. Reed, et al.; Phytochemistry 66, 2248 (2005)
- Allosteric regulation of tryptophan synthase channeling: the internal aldimine probed by trans-3-indole-3'-acrylate binding: P. Casino, et al.; Biochemistry 46, 7728 (2007)
- Structural insight into the inhibition of human kynurenine aminotransferase I/glutamine transaminase KII: Q. Han, et al.; J. Med. Chem. 52, 2786 (2009)
- Tryptophan 2,3-dioxygenase (TDO) inhibitors. 3-(2-(pyridyl)ethenyl)indoles as potential anticancer immunomodulators: E. Dolusic, et al.; J. Med. Chem. 54, 5320 (2011)
- Discovery of indolyl acrylamide derivatives as human diacylglycerol acyltransferase-2 selective inhibitors: K. Lee, et al.; Org. Biomol. Chem. 11, 849 (2013)
- Synthesis, biological evaluation and molecular docking studies of trans-indole-3-acrylamide derivatives, a new class of tubulin polymerization inhibitors: S.N. Baytas, et al.; Bioorg. Med. Chem. 22, 3096 (2014)
- Indoleacrylic acid produced by commensal Peptostreptococcus species suppresses inflammation: M. Wlodarska, et al.; Cell Host & Microbe 22, 25 (2017)






