Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
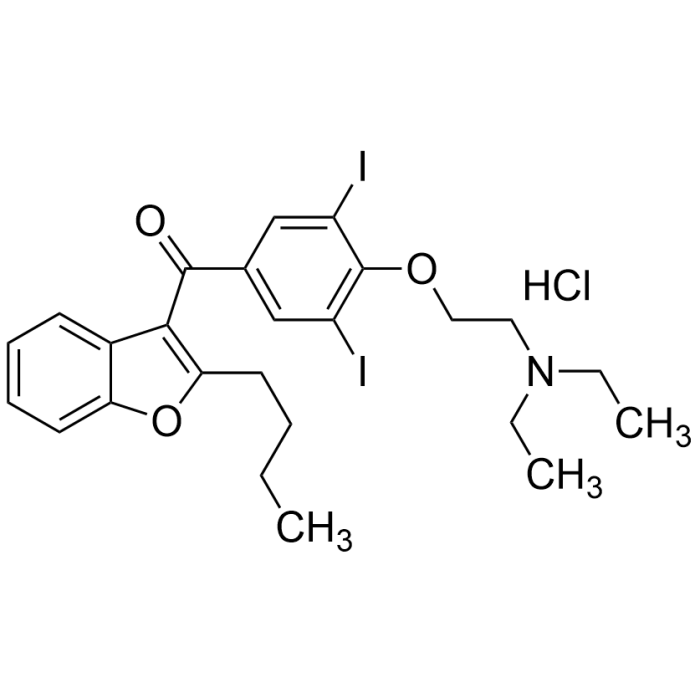
Amiodarone . HCl
| Product Details | |
|---|---|
| Synonyms | Cordarone; Pacerone; Nexterone; 2-Butyl-3-benzofuranyl 4-(2-(diethylamino)ethoxy)-3,5-diiodophenyl ketone hydrochloride |
| Product Type | Chemical |
| Properties | |
| Formula |
C25H29I2NO3 . HCl |
| MW | 645.3 . 36.5 |
| CAS | 19774-82-4 |
| RTECS | OB1361000 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥98% |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMF (10 mg/ml), DMSO (10 mg/ml) or ethanol (5 mg/ml). Only slightly soluble in water (<1mg/ml at 25 °C). |
| InChi Key | ITPDYQOUSLNIHG-UHFFFAOYSA-N |
| Smiles | O=C(C1=CC(I)=C(OCCN(CC)CC)C(I)=C1)C2=C(CCCC)OC3=CC=CC=C32.Cl |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Amiodarone hydrochloride is a non-selective ion channel blocker (potassium, sodium and calcium channels). Amiodarone is a class III antiarrhythmic agent, in that it prolongs both cardiac action potential and refractoriness by blocking potassium currents. It inhibits the voltage-gated potassium channel hERG, also known as KCNH2. In addition, amiodarone binds with high affinity to the σ-1 opioid receptor, 3-β-hydroxysteroid Δ8Δ7 isomerase, and C-8 sterol isomerase and inhibits human thyroid hormone receptors α and β. It also inhibits the cytochrome P450 (CYP) isoforms CYP2C8 and CYP3A4 in vitro at low micromolar concentrations.
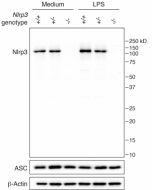
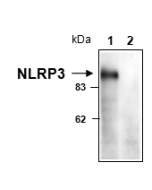
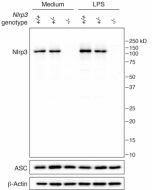
Amiodarone induces cell proliferation and myofibroblast differentiation via ERK1/2 and p38 MAPK signaling in fibroblasts. Recently, this K+ efflux inhibitor has been used to block the colocalisation of miniNLRP3 and ASC in red blood cells (RBCs), in a new process involving miniNLRP3 and RBC lysis, called spectosis.
- Inhibition of ATP-sensitive potassium channels of adult rat heart cells by antiarrhythmic drugs: R.A. Haworth, et al.; Circ. Res. 65, 1157 (1989)
- Therapeutic drug monitoring: Antiarrhythmic drugs: T.J. Campbell & K.M. Williams; Br. J. Clin. Pharmacol. 46, 307 (1998)
- Amiodarone inhibits cardiac ATP-sensitive potassium channels: D.S. Holmes, et al.; J. Cardiovasc. Electrophysiol. 11, 1152 (2000)
- Synthesis and preliminary characterization of a novel antiarrhythmic compound (KB130015) with an improved toxicity profile compared with amiodarone: B. Carlsson, et al.; J. Med. Chem. 45, 623 (2002)
- Mechanism-based inactivation of human cytochrome P4502C8 by drugs in vitro: T.M. Polasek, et al.; J. Pharmacol. Exp. Ther. 311, 996 (2004)
- Discovery of high-affinity ligands of σ1 receptor, ERG2, and emopamil binding protein by pharmacophore modeling and virtual screening: C. Laggner, et al.; J. Med. Chem. 48, 4754 (2005)
- Predicting hERG activities of compounds from their 3D structures: Development and evaluation of a global descriptors based QSAR model: N. Sinha & S. Sen; Eur. J. Med. Chem. 46, 618 (2011)
- Amiodarone induces cell proliferation and myofibroblast differentiation via ERK1/2 and p38 MAPK signaling in fibroblasts: J. Weng, et al.; Biomed. Pharmacother. 115, 108889 (2019)
- Amiodarone Advances the Apoptosis of Cardiomyocytes by Repressing Sigmar1 Expression and Blocking KCNH2-related Potassium Channels: H. Liang, et al.; Curr. Mol. Med. 25, 69 (2025)
- Red blood cells undergo lytic programmed cell death involving NLRP3: Y. Chen, et al.; Cell 188, 3013 (2025)