Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
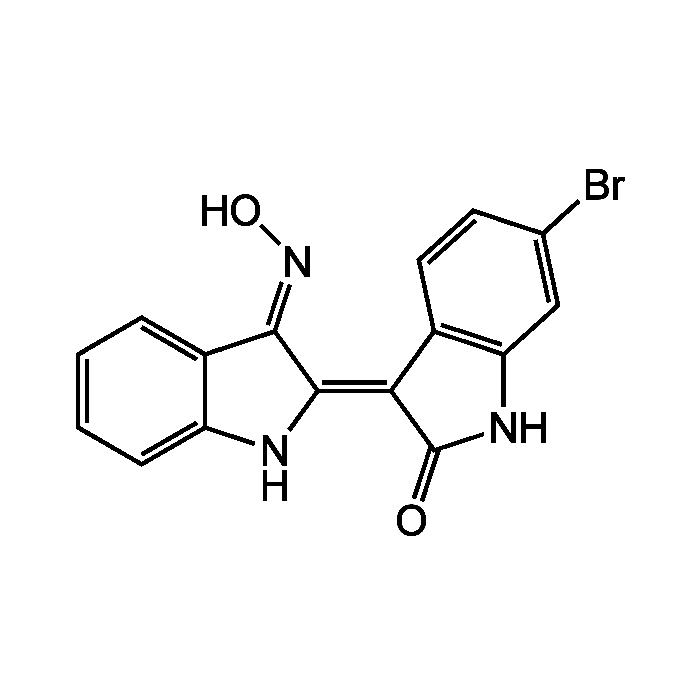
6BIO
As low as
160
CHF
CHF 160.00
In stock
Only %1 left
AG-MR-C0019-M01010 mgCHF 160.00
AG-MR-C0019-M05050 mgCHF 640.00
| Product Details | |
|---|---|
| Synonyms | BIO; 6-Bromoindirubin-3'-oxime |
| Product Type | Chemical |
| Properties | |
| Formula |
C16H10BrN3O2 |
| MW | 356.2 |
| CAS | 667463-62-9 |
| Purity Chemicals | ≥99% (NMR) |
| Appearance | Dark red solid. |
| Solubility | Soluble in DMSO or ethanol. |
| Reconstitution | Stock solutions can be made up to 10mM in DMSO. |
| Identity | Determined by 1H-NMR. |
| InChi Key | DDLZLOKCJHBUHD-WAVHTBQISA-N |
| Smiles | O\N=C1\C(\NC2=CC=CC=C\12)=C1\C(=O)NC2=C1C=CC(Br)=C2 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Potent, reversible and ATP-competitive glycogen synthase kinase-3α/β (GSK-3α/β) inhibitor.
- JAK/STAT3 signaling inhibitor.
- Phosphoinositide-dependent kinase 1 (PDK1) inhibitor.
- Anticancer compound.
- Potent antiproliferative agent. Suppresses metastasis.
- Apoptosis inducer.
- Sustains pluripotency and self-renewal of human and mouse embryonic stem cells (ESCs) by activation of the Wnt signaling pathway.
- Anti-leishmanial (IC50 = 0.150μM).
- Proto-oncogene tyrosine-protein kinase receptor RET inhibitor (IC50=0.51μM).
- Inhibits HIV-1 transcription and protects against Tat induced neurotoxicity.
Product References
- GSK-3-selective inhibitors derived from Tyrian purple indirubins: L. Meijer, et al.; Chem. Biol. 10, 1255 (2003)
- Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases: P. Polychronopoulos, et al.; J. Med. Chem. 47, 935 (2004)
- Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK-3-specific inhibitor: N. Sato, et al.; Nat. Med. 10, 55 (2004)
- The GSK-3 inhibitor BIO promotes proliferation in mammalian cardiomyocytes: A.S. Tseng, et al.; Chem. Biol. 13, 957 (2006)
- 7-Bromoindirubin-3'-oxime induces caspase-independent cell death: J. Ribas, et al.; Oncogene 25, 6304 (2006)
- Indirubin, the red shade of indigo: L. Meijer, et al. (Editors): In «Life in Progress», Station Biologique, Roscoff, 297 pp. (2006)
- Indirubin derivatives inhibit malignant lymphoid cell proliferation: A. Chebel, et al.; Leuk. Lymphoma 50, 2049 (2009)
- 6-Br-5methylindirubin-3'oxime (5-Me-6-BIO) targeting the leishmanial glycogen synthase kinase-3 (GSK-3) short form affects cell-cycle progression and induces apoptosis-like death: Exploitation of GSK-3 for treating leishmaniasis: E. Xingi, et al.; Int. J. Parasit. 39, 1289 (2009)
- Anticancer effects and antimetastatic mechanisms of novel indirubin derivatives: C.A. Kressirer; Diss. Ludwig-Maximilians-Universitaet Munchen, (2010)
- 6-Bromoindirubin-3'-Oxime Inhibits JAK/STAT3 Signaling and Induces Apoptosis of Human Melanoma Cells: L. Liu, et al.; Cancer Res. 71, 3972 (2011)
- Inhibition of Tat-mediated HIV-1 replication and neurotoxicity by novel GSK3-beta inhibitors: K. Kehn-Hall, et al.; Virology 415, 56 (2011)
- Induction of discrete apoptotic pathways by bromo-substituted indirubin derivatives in invasive breast cancer cells: K.A. Nicolaou, et al.; BBRC 425, 76 (2012)
- From Tyrian purple to kinase modulators: naturally halogenated indirubins and synthetic analogues: K. Vougogiannopoulou & A.L. Skaltsounis; Planta Med. 78, 1518 (2012)
- Pleiotrophin suppression of receptor protein tyrosine phosphatase-β/ζ maintains the self-renewal competence of fetal human oligodendrocyte progenitor cells: C.R. McClain, et al.; J. Neurosci. 32, 15066 (2012)
- Novel inverse binding mode of indirubin derivatives yields improved selectivity for DYRK kinases: V. Myrianthopoulos, et al.; ACS Med. Chem. Lett 4, 22 (2013)
- Indirubin Derivative 6BIO Suppresses Metastasis: S. Braig, et al.; Cancer Res. 73, 6004 (2013)








