Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
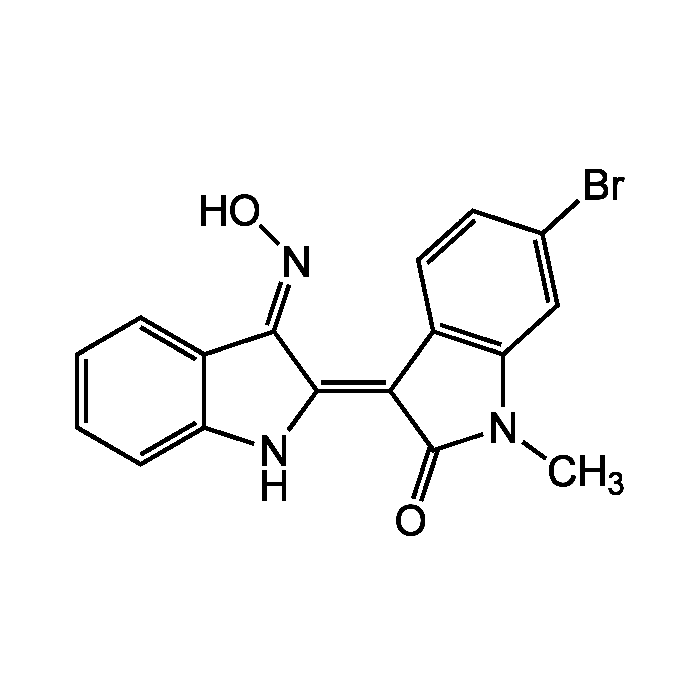
Me6BIO
As low as
130
CHF
CHF 130.00
In stock
Only %1 left
AG-MR-C0021-M0055 mgCHF 130.00
| Product Details | |
|---|---|
| Synonyms | MeBIO; 1-Methyl-6-bromo-indirubin-3'-oxime |
| Product Type | Chemical |
| Properties | |
| Formula |
C17H12BrN3O2 |
| MW | 370.2 |
| CAS | 667463-95-8 |
| Purity Chemicals | ≥95% (NMR) |
| Appearance | Dark red solid. |
| Solubility | Soluble in DMSO or ethanol. |
| Reconstitution | Stock solutions can be made up to 10mM in DMSO. |
| Identity | Determined by 1H-NMR. |
| InChi Key | JUKQKURGZYNHFN-NVVUQHMZSA-N |
| Smiles | CN1C(=O)\C(=C2/NC3=CC=CC=C3/C/2=N\O)C2=C1C=C(Br)C=C2 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- N-methylated inactive control analog of 6-bromoindirubin-3'-oxime (6BIO) (Prod. No. MR-C0019).
- Displays minimal activity against CDK1/Cyclin B, GSK-3 α/β and CDK5/p25.
- Aryl hydrocarbon receptor (AhR) agonist; causes redistribution of AhR to the nucleus.
Product References
- GSK-3-selective inhibitors derived from Tyrian purple indirubins: L. Meijer, et al.; Chem. Biol. 10, 1255 (2003)
- Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases: P. Polychronopoulos, et al.; J. Med. Chem. 47, 935 (2004)
- Independent actions on cyclin-dependent kinases and aryl hydrocarbon receptor mediate the antiproliferative effects of indirubins: M. Knockaert, et al.; Oncogene 23, 4400 (2004)
- The GSK-3 inhibitor BIO promotes proliferation in mammalian cardiomyocytes: A.S. Tseng, et al.; Chem. Biol. 13, 957 (2006)
- Indirubin, the red shade of indigo: L. Meijer, et al. (Editors): In «Life in Progress», Station Biologique, Roscoff, 297 pp. (2006)
- Indirubins decrease glioma invasion by blocking migratory phenotypes in both the tumor and stromal endothelial cell compartments: S.P. Williams, et al.; Cancer Res. 71, 5374 (2011)
- Pleiotrophin suppression of receptor protein tyrosine phosphatase-β/ζ maintains the self-renewal competence of fetal human oligodendrocyte progenitor cells: C.R. McClain, et al.; J. Neurosci. 32, 15066 (2012)