Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
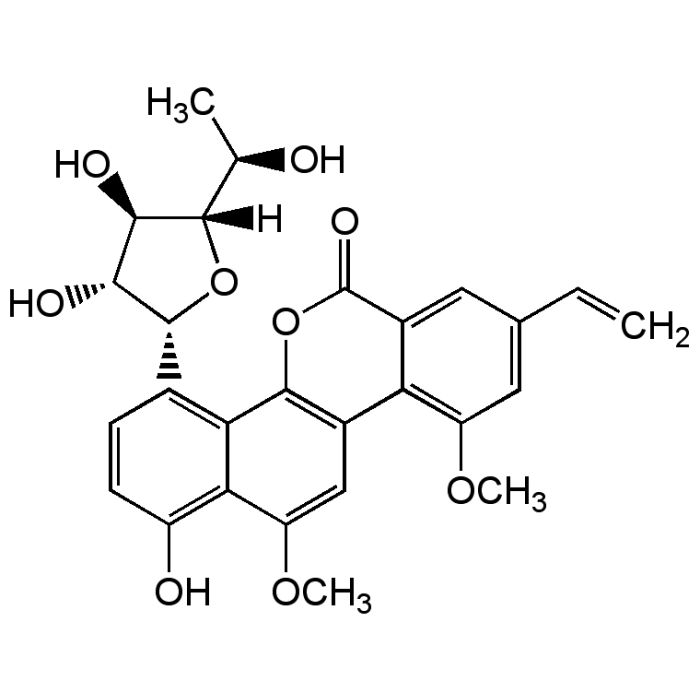
Gilvocarcin V
As low as
90
CHF
CHF 90.00
In stock
Only %1 left
BVT-0256-C100100 µgCHF 90.00
BVT-0256-C250250 µgCHF 145.00
BVT-0256-M0011 mgCHF 370.00
| Product Details | |
|---|---|
| Synonyms | Anandimycin A; Toromycin A; NSC 338943; DC-38-V; Antibiotic 1072B |
| Product Type | Chemical |
| Properties | |
| Formula |
C27H26O9 |
| MW | 494.5 |
| CAS | 77879-90-4 |
| RTECS | DI2240000 |
| Source/Host Chemicals | Isolated from Streptomyces sp. Gö 3592. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Yellow solid. |
| Solubility | Soluble in DMSO. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | XCWHINLKQMCRON-UCDARZNSSA-N |
| Smiles | [H][C@]1(O[C@@H]([C@H](O)[C@H]1O)C1=C2C3=C(C=C(OC)C2=C(O)C=C1)C1=C(OC)C=C(C=C)C=C1C(=O)O3)[C@@H](C)O |
| Shipping and Handling | |
| Shipping | BLUE ICE |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light when in solution. |
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. Stock solutions are stable for at least 3 months when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Antibiotic.
- Antitumor compound.
- Weakly active against Gram-positive bacteria and fungi.
- Mediates a unique cross-linking reaction between DNA and histone H3 by light.
- Single strand scission and covalent binding to DNA after photoactivation.
Product References
- Gilvocarcins, new antitumor antibiotics. 1. Taxonomy, fermentation, isolation and biological activities: H. Nakano, et al.; J. Antibiot. (Tokyo) 34, 266 (1981)
- Activation of antitumor agent gilvocarcins by visible light: R.K. Elespuru and S.K. Gonda; Science 223, 69 (1984)
- Photosensitized DNA breaks and DNA-to-protein crosslinks induced in human cells by antitumor agent gilvocarcin V: M.J. Peak, et al.; Chem. Biol. Interact. 67, 267 (1988)
- Biochemical characterisation of elsamicin and other coumarin-related antitumour agents as potent inhibitors of human topoisomerase II: A. Lorico & B. H. Long; Eur. J. Cancer 29A, 1985 (1993)
- Photophysical properties of gilvocarcins V and M and their binding constant to calf thymus DNA: R. Oyola, et al.; Photochem. Photobiol. 65, 802 (1997)
- Histone H3 and heat shock protein GRP78 are selectively cross-linked to DNA by photoactivatedgilvocarcin V in human fibroblasts: A. Matsumoto and P.C. Hanawalt; Cancer Res. 60, 3921 (2000)
- The complete gene cluster of the antitumor agent gilvocarcin V and its implication for the biosynthesis of the gilvocarcins: C. Fischer, et al.; J. Am. Chem. Soc. 125, 7818-7819 (2003)
- Multi-oxygenase complexes of the gilvocarcin and jadomycin biosyntheses: M. K. Kharel, et al.; J. Am. Chem. Soc. 129, 3780-3781 (2007)
- Inactivation of the ketoreductase gilU gene of the gilvocarcin biosynthetic gene cluster yields new analogues with partly improved biological activity: T. Liu, et al.; Chem Bio Chem 10, 278-286 (2009)
- Total synthesis of the antitumor natural product polycarcin V and evaluation of its DNA binding profile: X. Cai, et al.; Org. Lett. 16, 2962 (2014)