Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
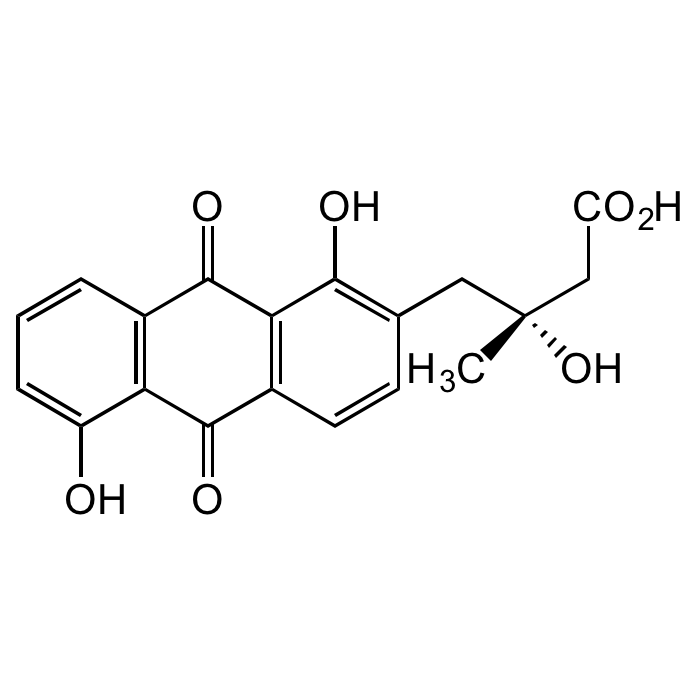
Fridamycin E
As low as
110
CHF
CHF 110.00
In stock
Only %1 left
BVT-0472-M0011 mgCHF 110.00
BVT-0472-M0055 mgCHF 325.00
| Product Details | |
|---|---|
| Synonyms | 9,10-Dihydro-β,1,5-trihydroxy-β-methyl-9,10-dioxo-2-anthracenebutanoic acid |
| Product Type | Chemical |
| Properties | |
| Formula |
C19H16O7 |
| MW | 356.3 |
| CAS | 116120-54-8 |
| Source/Host Chemicals | Isolated from Streptomyces parvulus. |
| Purity Chemicals | ≥98% (HPLC, NMR) |
| Appearance | Yellow to orange crystals. |
| Solubility | Soluble in DMSO. Sparingly soluble in methanol or acetone. |
| Identity | Determined by 1H-NMR and MS. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | MVEDBMGRQONWSQ-LJQANCHMSA-N |
| Smiles | C[C@](O)(CC(O)=O)CC1=C(O)C2=C(C=C1)C(=O)C1=C(C=CC=C1O)C2=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light. |
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. Store solutions at -20°C in the dark. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Anthraquinone derivative derived from an angucyclinone precursor.
- Antibiotic.
- Antibacterial agent.
Product References
- Fridamycine, Anthracyclin-analoge Antibiotica: P. Knicke; Ph. D. Thesis Univ. Goettingen (1984)
- Synthesis of rac- and ent-fridamycin E: K. Krohn & W. Baltus; Tetrahedron 44, 49 (1988)
- Synthetic anthracyclines from anthraquinones: R. Cambie, et al.; Austr. J. Chem. 45, 483 (1992)
- C-Aryl glycosides via tandem intramolecular benzyne-furan cycloadditions. Total synthesis of vineomycine B2 methyl ester: C.-L. Chen, et al.; JACS 128, 13696 (2006)
- A chemoenzymatic, enantioconvergent, asymmetric total synthesis of (R)-fridamycin E: B.J. Ueberbacher, et al.; Eur. J. Org. Chem. 7, 1266 (2005)
- Gephyromycin, the first bridged angucyclinone, from streptomyces griseus strain NTK 14: G. Bringmann, et al.; Phytochemistry 66, 1366 (2005)
- De novo asymmetric synthesis of fridamycin E: Q. Chen, et al.; Org. Lett. 13, 6592 (2011)
- Angucyclines from an insect-derived actinobacterium Amycolatopsis sp. HCa1 and their cytotoxic activity: Z.K. Guo, et al.; Bioorg. Med. Chem. Lett. 22, 7490 (2012)