Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
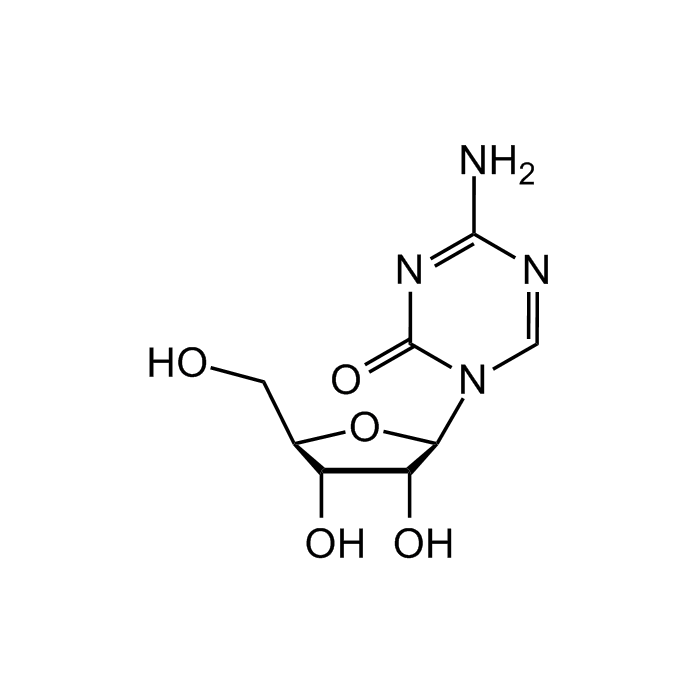
5-Azacytidine
| Product Details | |
|---|---|
| Synonyms | 5-Azacitidine; Antibiotic U18496; 5-AzaC; Ladakamycin; Mylosar; NSC102816; NSC103-627; U18496; WR183027 |
| Product Type | Chemical |
| Properties | |
| Formula |
C8H12N4O5 |
| MW | 244.20 |
| CAS | 320-67-2 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in ethanol, DMSO, DMF (30mg/ml), water or PBS (10mg/ml). |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | NMUSYJAQQFHJEW-KVTDHHQDSA-N |
| Smiles | OC[C@@H]1[C@@H](O)[C@@H](O)[C@H](N2C=NC(N)=NC2=O)O1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
5-Azacytidine, a chemical analogue of the DNA and RNA nucleoside cytidine, is an cell permeable inhibitor of DNA methyltransferases, potentially serving to reverse epigenetic changes. It reduces hypermethylation associated with certain diseases, including myelodysplastic syndromes (IC50s = 2.4 and 2.6μM for in vitro anti-myeloma activity) and cancer (IC50s ~ 0.4μM for inhibiting proliferation of various cancer cell lines). It has a reported half-life of 17 hours and is considerably cytotoxic; it must be incorporated into DNA to covalently trap DNA methyltransferases. Induces demethylation and reactivation of silenced genes. Improves the efficiency of reprogramming of stem cells; induces differentiation of mesenchymal stem cells into cardiomyocytes.
(1) H. Kusaba, et al.; Eur. J. Biochem. 262, 924 (1999) | (2) B. Brueckner, et al.; Cancer Res. 65, 6305 (2005) | (3) F. Lyko & R. Brown; J. Natl. Cancer Inst. 97, 1498 (2005) | (4) C. Stresemann, et al.; Cancer Res. 66, 2794 (2006) | (5) M. Esteller; N. Engl. J. Med. 358, 1148 (2008) | (6) T.S. Mikkelsen, et al.; Nature 454, 49 (2008) | (7) A.M. Giraldo, et al.; Methods Mol. Biol. 791, 145 (2011) | (8) J.J. Chong, et al.; Cell Res. 22, 1932 (2013) | (9) M. Bhuvanagiri, et al.; EMBO Mol. Med. 6, 1593 (2014)