Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
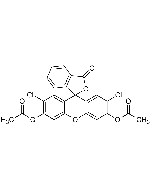
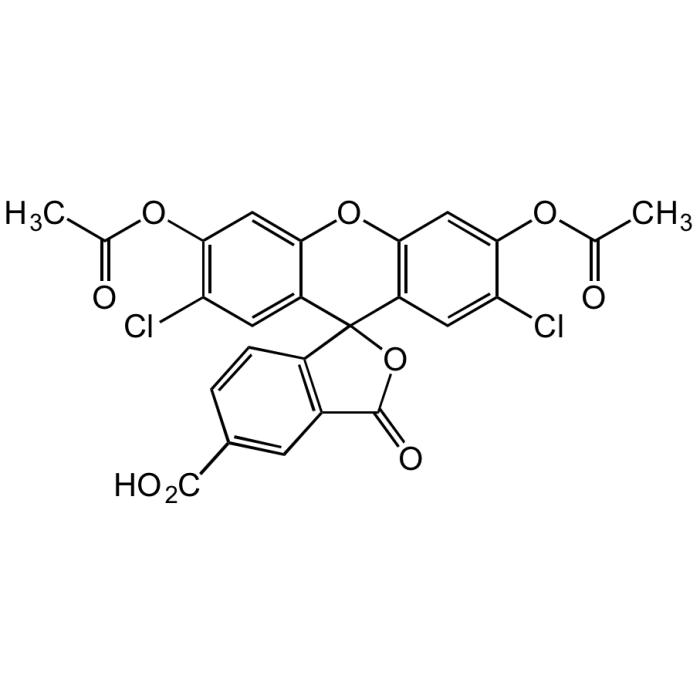
5-Carboxy-DCFDA
| Product Details | |
|---|---|
| Synonyms | 5-CDCFDA; 5-Carboxy-2',7'-dichlorofluorescein diacetate; 5-Carboxy-2',7'-dichlorofluorescein DA |
| Product Type | Chemical |
| Properties | |
| Formula | C25H14Cl2O9 |
| MW | 529.28 |
| CAS | 144489-09-8 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥95% (NMR) |
| Appearance | Off-white powder. |
| Solubility | Soluble in DMSO, DMF, acetonitrile, methanol or chloroform. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | AJIVVDSJIOOZOJ-UHFFFAOYSA-N |
| Smiles | ClC1=C(OC(C)=O)C=C2C(C3(C(C=CC(C(O)=O)=C4)=C4C(O3)=O)C(C=C(Cl)C(OC(C)=O)=C5)=C5O2)=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Non-activated, amine-reactive fluorescent probe. Single 5-isomer of CDCFDA that can be used in biomolecules conjugation. CDCFDA is an amine–reactive fluorescein diacetate (FDA) derivative used to prepare a variety of FDA conjugates. CDCFDA is a non-fluorescent molecule that diffuse into cells and is hydrolyzed by intracellular non-specific esterases, yielding a fluorescent probe. The probe accumulates exclusively in viable cells with intact cell membranes. CFCFDA does not stain dead cells. CDCFDA labeling can be used for monitoring cells by flow cytometry or fluorescence microscopy. The probe is also frequently used to monitor reactive oxygen species (ROS) generation in live cells and intracellular pH levels. Spectral data: λex=504nm, λem=529nm.
(1) C.K. Raymond et al.; J. Cell Biol. 111, 877 (1990) | (2) J.P. Kehrer & T. Paraidathathu; Free Rad. Res. Commun. 16, 217 (1992) | (3) Q.P. Lloyd, et al.; J. Biol. Chem. 270, 22445 (1995) | (4) A.L. Inselman, et al.; Curr. Microbiol. 38, 295 (1999) | (5) M.J. Zamek-Gliszczynski, et al.; J. Pharmacol. Exp. Therap. 304, 801 (2003) | (6) L. Li, et al.; Free Radic. Res. 42, 354 (2008) | (7) M. Medhora, et al.; Am. J. Physiol. Lung Cell Mol. Physiol. 294, L902 (2008) | (8) S. Siissalo, et al.; Eur. J. Pharm. Biopharm. 71, 332 (2009) | (9) A.I. Pogue, et al.; Int. J. Mol. Sci. 13, 9615 (2012) | (10) J. Roh, et al.; Redox Biol. 11, 254 (2016) | (11) J.L. Roh, et al.; Cancer Lett. 381, 96 (2016)