Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
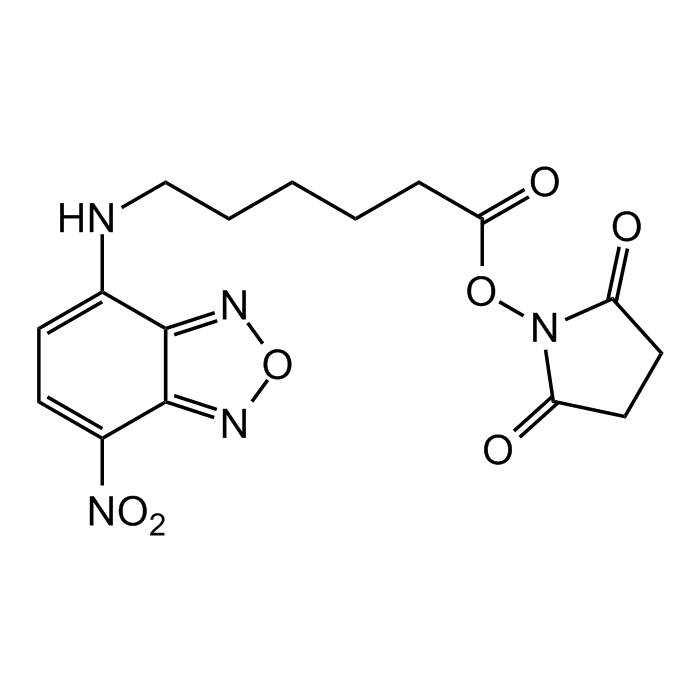
NBD-X SE
| Product Details | |
|---|---|
| Synonyms | NBD-X succinimidyl ester; NBD-C6 SE; 6-(7-Nitrobenzofurazan-4-ylamino)hexanoic acid NHS ester |
| Product Type | Chemical |
| Properties | |
| Formula |
C16H17N5O7 |
| MW | 391.33 |
| CAS | 145195-58-0 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Orange powder. |
| Solubility | Soluble in DMSO or DMF. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | ULIVORQJLJKPHQ-UHFFFAOYSA-N |
| Smiles | O=C(ON1C(CCC1=O)=O)CCCCCNC2=CC=C([N+]([O-])=O)C3=NON=C32 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
NBD-X, SE is an excellent amino-reactive building block that can be used to prepare peptide conjugates and other bioconjugates/biopolymers. It has much higher conjugation efficiency than NBD-Cl and NBD-F. This amine-reactive NBD derivative, containing a convenient succinimidyl ester, can be used to develop environmentally sensitive bioconjugates. NBD is a functional analog of the dinitrophenyl hapten, and its fluorescence is quenched upon binding to anti-dinitrophenyl (anti-DNP) antibodies. The X-spacer separates the fluorescent dye from the protein allowing greater sensitivity and reduced perturbation effects on protein labeling. Spectral properties: lamdaEx=460; lambdaEm=540.
(1) C. Sherrill, et al.; Biochemistry 34, 3553 (1995) | (2) B.K. Nunnally, et al.; Anal. Chem. 69, 2392 (1997) | (3) J.T. Elliott & G.D. Prestwich; Bioconjug. Chem. 11, 832 (2000) | (4) S. Ozaki, et al.; PNAS 97, 11286 (2000) | (5) A. Karlstrom, et al.; Anal. Biochem. 295, 22 (2001) | (6) F. Eisele, et al.; Chem. Eur. J. 8, 3362 (2002) | (7) L. Silva, et al.; J. Photochem. Photobiol. B 72, 17 (2003) | (8) J.M. Bergen, et al.; Bioconjug. Chem. 19, 377 (2008)