Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
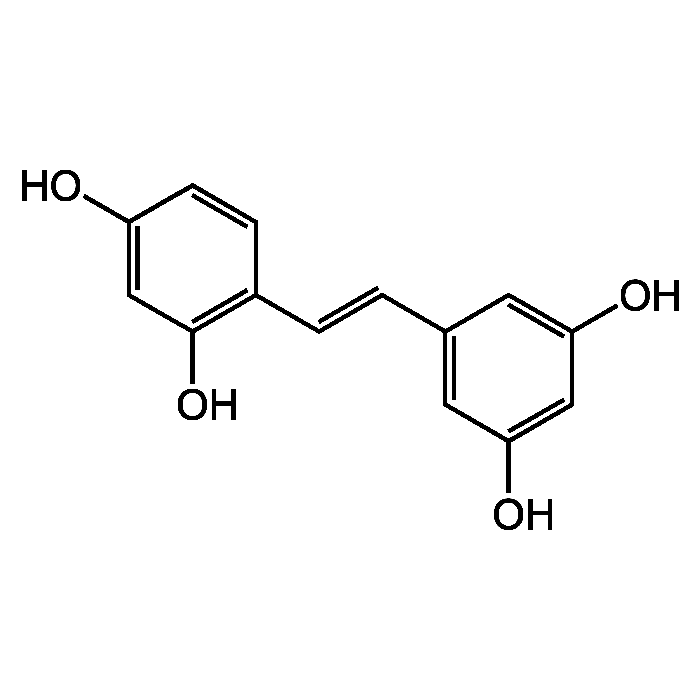
Oxyresveratrol
| Product Details | |
|---|---|
| Synonyms | trans-2',3,4',5-Tetramethoxystilbene |
| Product Type | Chemical |
| Properties | |
| Formula | C14H12O4 |
| MW | 244.2 |
| CAS | 29700-22-9 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in chloroform. |
| Identity | Determined by NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | PDHAOJSHSJQANO-UPHRSURJSA-N |
| Smiles | OC1=CC(O)=C(\C=C\C2=CC(O)=CC(O)=C2)C=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Naturallly occuring analog of resveratrol. Potent antioxidant and free-radical scavenger. Shown to have neuroprotective activity against cerebral ischemia and against traumatic injury. Apoptosis inhibitor in transient cerebral ischemia. Inhibits viral DNA replication and late viral protein synthesis of African Swine fever virus. Has depigmenting effects by effectively inhibiting tyrosinase activity, which catalyzes the rate-limiting step in synthesizing melanin pigments. Has anti-inflammatory properties based on inhibition of iNOS expression through down-regulation of NF-kB binding activity and significant inhibition of COX-2 activity. Antihyperlipidemic agent.
(1) Y.M. Kim, et al.; J. Biol. Chem. 277, 16340 (2002) | (2) P. Lorenz, et al.; Nitric Oxide 9, 64 (2003) | (3) K.-O. Chung, et al.; J. Pharm. Pharmacol. 55, 1695 (2003) | (4) S.A. Andrabi, et al.; Brain Res. 1017, 98 (2004) | (5) I. Galindo, et al.; Antiviral Res. 91, 57 (2011) | (6) M. Chatsumpun, et al.; Nat. Prod. Commun. 6, 41 (2011) | (7) J.T. Weber, et al.; Eur. J. Pharmacol. 680, 55 (2012) | (8) S.-P. Jo, et al.; Food Chem. Toxicol. 65, 213 (2014)