Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chimerigen
IL-1α (human) (rec.)

| Product Details | |
|---|---|
| Synonyms | Interleukin-1α; IL-1F1; IL-1A |
| Product Type | Protein |
| Properties | |
| Source/Host | HEK 293 cells |
| Sequence | Human IL-1α (aa 113-271). |
| Crossreactivity | Human |
| Biological Activity | The ED50 as determined by the dose-dependent stimulation of murine D10S cells is ≤ 0.001 ng/ml, corresponding to a specific activity of ≥ 1 x 109units/mg. |
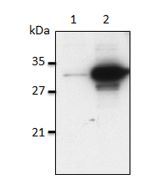
| MW | ~19kDa (SDS-PAGE) |
| Purity | ≥95% (SDS-PAGE) |
| Endotoxin Content | <0.01EU/μg protein (LAL test; Lonza). |
| Reconstitution |
Reconstitute 10µg vial with 100 µl sterile water to a concentration of 0.1mg/ml. Reconstitute 50µg vial with 100 µl sterile water to a concentration of 0.5mg/ml. Add 1X PBS to the desired protein concentration. |
| Formulation | Lyophilized from 0.2μm-filtered solution in PBS. |
| Other Product Data | NCBI reference NP_000566.3: IL-1α (human) |
| Declaration | Manufactured by Chimerigen. |
| Shipping and Handling | |
| Shipping | BLUE ICE |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Avoid freeze/thaw cycles. Centrifuge lyophilized vial before opening and reconstitution. PBS containing at least 0.1% BSA should be used for further dilutions. |
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. Working aliquots are stable for up to 3 months when stored at -20°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
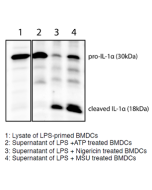
The most prominent members of the interleukin-1 (IL-1) superfamily are IL-1α and IL-1β. They lack a signal peptide and are secreted by an unconventional, endoplasmic reticulum-Golgi-independent mechanism. IL-1α was reported to be more widely and constitutively expressed and has intracellular functions, but also acts locally in a membrane-bound form by activating IL-1R1. Additionally, passive release of IL-1α upon cell death can trigger a sterile inflammatory response to dying cells. The cleavage of IL-1α is not mediated by caspase-1 and is not required for binding to IL-1R1. Recently it has been observed that all activators of the inflammasome NLRP3/NALP3 induce the simultaneous secretion of IL-1α and IL-1β. Although most activators fully rely on the inflammasome for IL-1α secretion, some induce the processing and secretion of IL-1α in an inflammasome-independent manner.