Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
anti-FADD (human), mAb (1C4)

| Product Details | |
|---|---|
| Synonyms | FAS-associated Death Domain Protein; Growth-inhibiting Gene 3 Protein; Mediator of Receptor Induced Toxicity; MORT1 |
| Product Type | Monoclonal Antibody |
| Properties | |
| Clone | 1C4 |
| Isotype | Mouse IgG1 |
| Source/Host | Purified from concentrated hybridoma tissue culture supernatant. |
| Immunogen/Antigen | Recombinant human FADD. |
| Application |
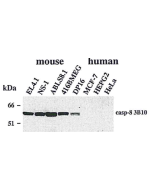
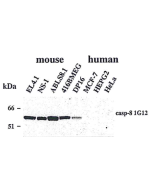
ELISA Western Blot: (1μg/ml) Immunoprecipitation |
| Crossreactivity | Human |
| Specificity |
Recognizes the C-terminus of human FADD only in its unphosphorylated form. |
| Purity | ≥95% (SDS-PAGE) |
| Purity Detail | Protein G-affinity purified. |
| Concentration | 1 mg/ml |
| Formulation | Liquid. In PBS and 0.02% sodium azide. |
| Isotype Negative Control | |
| Other Product Data |
UniProt link Q13158: FADD (human) |
| Accession Number | Q13158 |
| Shipping and Handling | |
| Shipping | BLUE ICE |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
After opening, prepare aliquots and store at -20°C. Avoid freeze/thaw cycles. |
| Use/Stability | Stable for at least 1 year after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
FADD (FAS-associated death domain protein) is an apoptotic adaptor molecule that recruits caspase-8 or caspase-10 to the activated Fas (CD95) or TNFR-1 receptors. Procaspase-8 belongs to the family of caspases. Binding of FasL to Fas leads to formation of a receptor complex at the cellular membrane, which was named DISC. The DISC consists of oligomerized receptors, the DD-containing adaptor molecule FADD, procaspase-8, procaspase-10 and c-FLIP. The DISC structure provides a platform for the oligomerization of procaspase-8 that allows two procaspase-8 homodimers to be in the close proximity leading to the initial activation of procaspase-8. FADD is also involved in interferon-mediated antiviral immune response and functions in the positive regulation of interferon signaling. FADD also plays a role in embryonic development and the cell cycle reentry of T cells.
- DEDD, a novel death effector domain-containing protein, targeted to the nucleolus: A.H. Stegh, et al.; EMBO J. 17, 5974 (1998)
- Phosphorylation of FADD/ MORT1 at serine 194 and association with a 70-kDa cell cycle-regulated protein kinase: C. Scaffidi, et al.; J. Immunol. 164, 1236 (2000)
- Phosphorylation of FADD at Serine 194 by CKIα Regulates Its Nonapoptotic Activities: E.C. Alappat, et al.; Mol. Cell 19, 321 (2005)
- CD95 Stimulation Results in the Formation of a Novel Death Effector Domain Protein-containing Complex: I.N. Lavrik, et al.; J. Biol. Chem. 283, 26401 (2008)
- A New C-Terminal Cleavage Product of Procaspase-8, p30, Defines an Alternative Pathway of Procaspase-8 Activation: J.C. Hoffmann, et al.; Mol. Cell. Biol. 29, 4431 (2009)
- Caspase-8 activity has an essential role in CD95/Fas-mediated MAPK activation: A.M.M. Kober, et al.; Cell Death Dis. 2, e212 (2011)
- Modulation of the CD95-Induced Apoptosis: The Role of CD95 N-Glycosylation: O.M. Shatnyeva, et al.; PLoS One 6, e19927 (2011)
- Quantification of High-Molecular Weight Protein Platforms by AQUA Mass Spectrometry as Exemplified for the CD95 Death-Inducing Signaling Complex (DISC): U. Warnken, et al.; Cells 2, 476 (2013)
- Targeting Thioredoxin-1 by dimethyl fumarate induces ripoptosome-mediated cell death: A. Schroeder, et al.; Sci. Rep. 7, 43168 (2017)
- A Dual Role of Caspase-8 in Triggering and Sensing Proliferation-Associated DNA Damage, a Key Determinant of Liver Cancer Development: Y. Boege, et al.; Cancer Cell 32, 342 (2017)
- Delineating the role of c-FLIP/NEMO interaction in the CD95 network via rational design of molecular probes: N.V. Ivanisenko, et al.; BMC Genomics 20, 293 (2019)
- Evaluation of CDK9 Inhibition by Dinaciclib in Combination with Apoptosis Modulating izTRAIL for the Treatment of Colorectal Cancer: X. Shen, et al.; Biomed. 11, 928 (2023)