Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
SARS-CoV-2 Neutralizing Antibodies Detection Kit (B.1.351 Variant, Beta)

| Product Details | |
|---|---|
| Synonyms | SARS-CoV-2 Neutralizing Antibodies Detection Kit (B.1.351 Variant, South Africa); COVID-19 Neutralizing Antibodies Detection Kit (PB.1.351 Variant, Beta); 2019-nCoV Spike Protein S1 (RBD) Neutralizing Antibodies Detection Kit (B.1.351 Variant, Beta) |
| Product Type | Kit |
| Properties | |
| Application Set | Qualitative ELISA |
| Specificity |
To measure the presence of neutralizing/blocking antibodies in human serum/plasma that inhibit the binding of the SARS-CoV-2 Spike (RBD) (B.1.351 Variant, Beta) protein to its human receptor ACE2. |
| Crossreactivity |
Avian Bovine Goat Guinea pig Human Monkey Mouse Pig Rabbit Rat |
| Quantity |
Contains sufficient materials for 96 reactions |
| Sample Type |
Plasma Serum |
| Detection Type | Colorimetric |
| Shipping and Handling | |
| Shipping | BLUE ICE |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice |
After reconstitution, prepare aliquots and store at -20°C. Avoid freeze/thaw cycles. Plate and reagents should reach room temperature before use. |
| Use/Stability | 12 months after the day of manufacturing. See expiry date on ELISA Kit box. |
| Documents | |
| Manual |
 Download PDF Download PDF |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
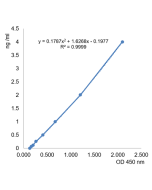
Coronaviruses (CoVs) are enveloped non-segmented positive-sense single-stranded RNA viruses and can infect respiratory, gastrointestinal, hepatic and central nervous system of human and many wild animals. Recently, a new severe acute respiratory syndrome β-coronavirus called SARS-CoV-2 has emerged, which causes an epidemic of acute respiratory syndrome (called coronavirus human disease 2019 or COVID-19). SARS-CoV-2 contains 4 structural proteins, including Envelope (E), Membrane (M), Nucleocapsid (N) and Spike (S), which is a transmembrane protein, composed of two subunits S1 and S2. The S1 subunit contains a receptor binding domain (RBD), which binds to the cell surface receptor Angiotensin-Converting Enzyme 2 (ACE2) present at the surface of epithelial cells, causing mainly infection of human respiratory cells.The SARS-CoV-2 Neutralizing Antibodies Detection Kit (B.1.351 Variant, Beta) contains key reagents required to test the presence of functional neutralizing antibodies against SARS-CoV-2 (variant B.1.351) present in the serum or plasma or/and to test the cross-reaction of SARS-CoV-2 neutralizing antibodies directed to previous variants. It is an easy and fast alternative to the classical neutralization assay using Vero E6 cells. This Detection Kit is based on a colorimetric reaction, which measures the binding of the RBD of the Spike S protein from SARS-CoV-2 to its human receptor ACE2. The presence of neutralizing/blocking antibodies in the samples are detected by reduction of signal indicating the inhibition of the Spike-ACE2 binding.
The new variant of SARS-CoV-2, called B.1.351 (Beta), was detected in South Africa. This variant carries three mutations in the RBD at the positions 417, 484 and 501 (K417N, E484K, N501Y) and is associated with a higher viral load, which may suggest potential for increased transmissibility.