Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
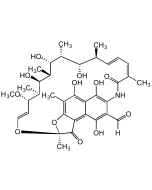
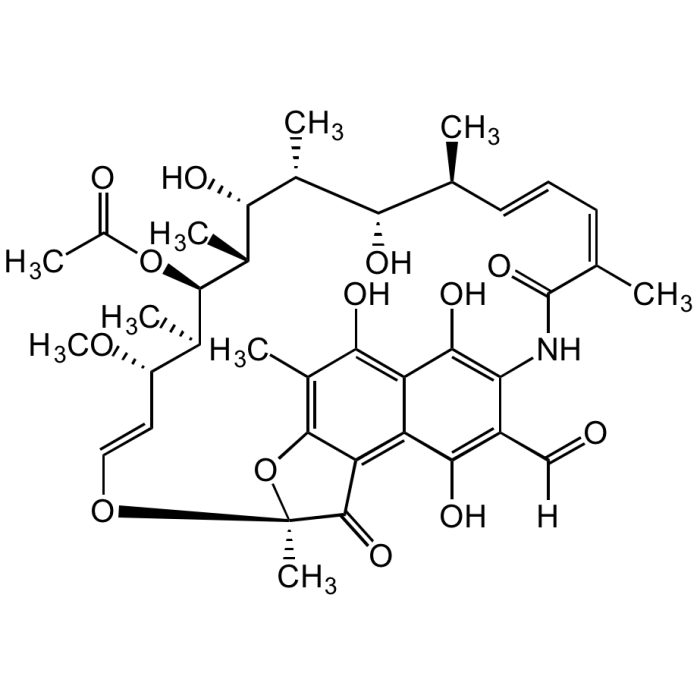
Rifamycin AF
As low as
90
CHF
CHF 90.00
In stock
Only %1 left
AG-CN2-0321-G0011 gCHF 90.00
AG-CN2-0321-G0055 gCHF 360.00
AG-CN2-0321-G01010 gCHF 660.00
| Product Details | |
|---|---|
| Synonyms | NCI 145-635; 3-Formylrifamycin; 3-Formylrifampicin SV; 3-Formylrifamycin SV; Rifaldehyde |
| Product Type | Chemical |
| Properties | |
| Formula |
C38H47NO13 |
| MW | 725.8 |
| CAS | 13292-22-3 |
| Source/Host Chemicals | Semisynthetic. |
| Purity Chemicals | ≥95% (HPLC) |
| Appearance | Red powder. |
| Solubility | Soluble in DMSO, aqueous acetonitrile or ethanol. |
| Identity | Determined by 1H-NMR and MS. |
| InChi Key | BBNQHOMJRFAQBN-LXFNAIAASA-N |
| Smiles | OC1=C(NC(/C(C)=C\C=C\[C@H](C)[C@H](O)[C@@H](C)[C@H]([C@@H](C)[C@@H]([C@H](C)[C@H](/C=C/O2)OC)OC(C)=O)O)=O)C(C([H])=O)=C(O)C3=C4C(O[C@@]2(C)C4=O)=C(C)C(O)=C31 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light. Protect from light when in solution. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Ansamycin antibiotic.
- Intermediate of rifampicin.
- Selective inhibitor of bacterial DNA-dependent RNA polymerase (RNAP).
- Effective against mycobacteria and therefore used in research of tuberculosis, leprosy and Mycobacterium avium complex (MAC) infections.
Product References
- Rifamycins: A General View: S. Riva & L.G. Silvestri; Ann. Rev. Microbiol. 26, 199 (1972)
- Spin-labeled rifamycin: biological activity: Z.Z. Raykov, et al.; Pharmazie 63, 61 (2008)
- New anti-tuberculosis agents amongst known drugs: K.A.E. Lougheed, et al.; Tuberculosis 89, 364 (2009)
- The effect of complexation of 3-formylrifamycin SV macrocyclic ether derivatives with metal cations and small nitrogen-containing organic molecules on antibacterial activity against S. aureus and S. epidermidis: P. Przybylski, et al.; Bioorg. Med. Chem. Lett. 25, 3903 (2015)
- Rifamycin antibiotics - new compounds and synthetic methods. Part 4: Study of the reaction of 3-formylrifamycin SV with secondary amines and ketones: K. Bujnowski, et al.; Tetrahedr. 71, 158 (2015)