Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
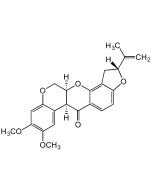
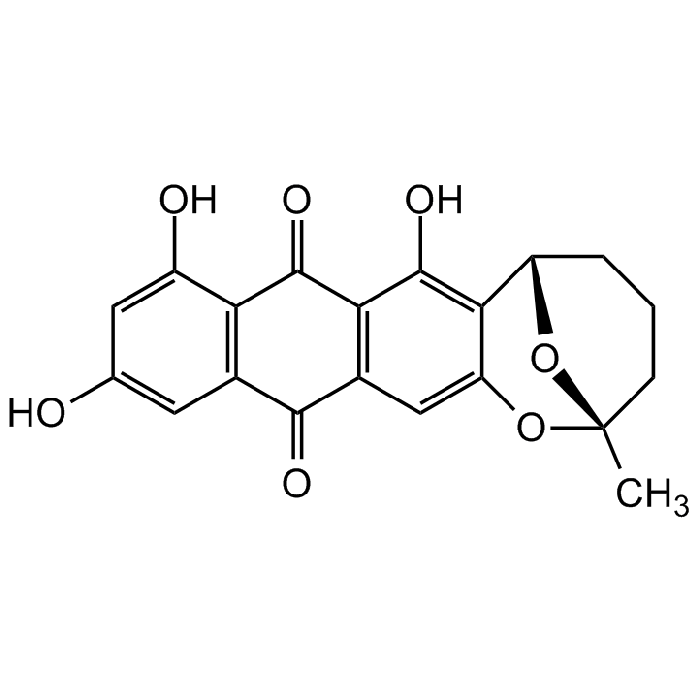
Averufin
As low as
180
CHF
CHF 180.00
In stock
Only %1 left
AG-CN2-0527-M0011 mgCHF 180.00
| Product Details | |
|---|---|
| Synonyms | (2S,6S)-7,9,11-Trihydroxy-2-methyl-3,4,5,6-tetrahydro-2H-2,6-epoxyanthra[2,3-b]oxocine-8,13-dione; BRN1299991 |
| Product Type | Chemical |
| Properties | |
| Formula |
C20H16O7 |
| MW | 368.3 |
| CAS | 14016-29-6 |
| RTECS | KC4150000 |
| Source/Host Chemicals | Isolated from fungus Aspergillus versicolor. |
| Purity Chemicals | ≥95% |
| Appearance | Yellow to orange solid. |
| Solubility | Soluble in DMSO (5mg/ml) or methanol (1mg/ml). |
| Identity | Determined by 1H-NMR. |
| InChi Key | RYFFZJHGQCKWMV-YUNKPMOVSA-N |
| Smiles | OC1=CC(O)=CC(C(C2=C3C(O)=C4C(O[C@@]5(C)CCC[C@@H]4O5)=C2)=O)=C1C3=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Keep cool and dry. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Mycotoxin. Biosynthetic precursor of aflatoxins.
- Antibiotic. Shows moderate antibacterial activity against Gram-positive bacteria.
- Oxidative phosphorylation (OXPHOS) inhibitor. Inhibits succinate cytochrome c reductase complex (III) of the respiratory chain in mitochondria.
- Useful tool for immunometabolism research.
Product References
- The Inhibition of Mitochondrial Respiration by Anthraquinone Mycotoxiins, Averufin and Versicolorins A, B: K. Kawai, et al.; Proc. Jpn. Assoc. Mycotoxicol. 1983, 35 (1983)
- Averufin, an anthraquinone mycotoxin possessing a potent uncoupling effect on mitochondrial respiration: K. Kawai, et al.; Appl. Environ. Microbiol. 47, 481 (1984)
- Genotoxicity of a variety of mycotoxins in the hepatocyte primary culture/DNA repair test using rat and mouse hepatocytes: H. Mori, et al.; Cancer Res. 44, 2918 (1984)
- Averufanin is an aflatoxin B1 precursor between averantin and averufin in the biosynthetic pathway: S.P. McCormick, et al.; Appl. Environ. Microbiol. 53, 14 (1987)
- Monoamine oxidase inhibitors from a fungus: Emericella navahoensis: M. Yamazaki, et al.; Chem. Pharm. Bull. 36, 670 (1988)
- Isolation, structure elucidation and biological activity of 8-O-methylaverufin and 1,8-O-dimethylaverantin as new antifungal agents from Penicillium chrysogenum: R.P. Maskey, et al.; J. Antibiot. 56, 459 (2003)
- The antifungal metabolites obtained from the rhizospheric Aspergillus sp. YIM PH30001 against pathogenic fungi of Panax notoginseng: K. Liu, et al.; Nat. Prod. Res. 28, 2334 (2014)
- Induction of Secondary Metabolites from the Marine-Derived Fungus Aspergillus versicolor through Co-cultivation with Bacillus subtilis: N.M. Abdel-Wahab, et al.; Planta Med. 85, 503 (2019)