Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
UBS109
As low as
90
CHF
CHF 90.00
In stock
Only %1 left
AG-CR1-3704-M0011 mgCHF 90.00
AG-CR1-3704-50015 x 1 mgCHF 190.00
AG-CR1-3704-M01010 mgCHF 320.00
| Product Details | |
|---|---|
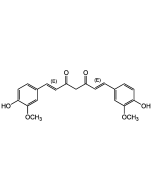
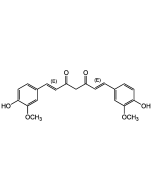
| Synonyms | (3E,5E)-1-Methyl-3,5-bis(2-pyridinylmethylene)-4-piperidinone; Monocarbonyl Analog of Curcumin |
| Product Type | Chemical |
| Properties | |
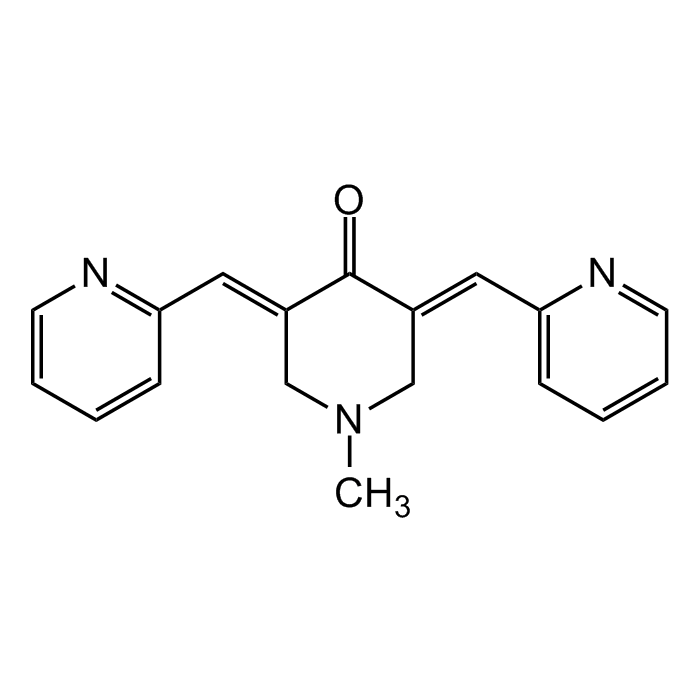
| Formula |
C18H17N3O |
| MW | 291.4 |
| CAS | 1258513-40-4 |
| Purity Chemicals | ≥95% (HPLC) |
| Appearance | Yellow solid. |
| Solubility | Soluble in DMSO (10mg/ml). Insoluble in water. |
| Identity | Determined by 1H-NMR. |
| Other Product Data |
Note: Always prepare and use fresh solution. We recommend to immediately use the fresh solution. In methanol and ethanol the compound decomposes rapidly. |
| InChi Key | VCLPNFMJSSBBKX-WFYKWJGLSA-N |
| Smiles | O=C(/C(CN(C)C/1)=C/C2=NC=CC=C2)C1=C\C3=CC=CC=N3 |
| Shipping and Handling | |
| Shipping | BLUE ICE |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Most water soluble synthetic monocarbonyl analog of curcumin (MAC) for in vitro and in vivo application.
- Potently inhibits NF-κB and its nuclear translocation by suppressing IKK-α and IKK-β, consequently leading to apoptosis.
- Potent DNA hypomethylating agent. Inhibits HSP90 and NF-κB leading to downregulation of DNA methyltransferase-1 (DNMT-1) expression.
- Cytotoxic against several cancers in vitro and in vivo. Shows excellent activity against xenografts of head and neck squamous cell carcinoma, pancreatic cancer, colon cancer and breast cancer.
- Antiangiogenic agent. Induces the downregulation of HIF-1α, HSP90, COX-2 and VEGF in tumor samples from xenograft models compared to untreated xenografts.
- Shows preventive effects on bone loss induced by breast cancer cell bone metastasis. Found to have a potential stimulating effect on osteoblastogenesis and a suppressive effect on osteoclastogenesis in vitro through Smad activation and NF-κB inhibition. May have promise in the development into an antiosteoporotic agent capable of promoting new bone formation while simultaneously reducing bone resorption.
Product References
- Novel curcumin analogue UBS109 potently stimulates osteoblastogenesis and suppresses osteoclastogenesis: involvement in Smad activation and NF-kB inhibition: M. Yamaguchi, et al.; Integr. Biol. 4, 905 (2012)
- Novel synthetic curcumin analogues EF31 and UBS109 are potent DNA hypomethylating agents in pancreatic cancer: G. Nagaraju, et al.; Cancer Lett. 341, 195 (2013)
- Curcumin analogue UBS109 prevents bone loss in breast cancer bone metastasis mouse model: involvement in osteoblastogenesis and osteoclastogenesis: M. Yamaguchi, et al.; Cell Tissue Res. 357, 245 (2014)
- Liver S9 Fraction-Derived Metabolites of Curcumin Analogue UBS109: T.W. Moore, et al.; Med. Chem. Lett. 5, 288 (2014)
- Curcumin analog UBS109 prevents bone marrow osteoblastogenesis and osteoclastogenesis disordered by coculture with breast cancer MDA-MB-231 bone metastatic cells in vitro: M. Yamaguchi, et al.; Mol. Cell Biochem. 401, 1 (2015)
- Eliminating the Heart from the Curcumin Molecule: Monocarbonyl Curcumin Mimics (MACs): D. Shetty, et al.; Molecules 20, 249 (2015) (Review)
- Inhibition of breast cancer metastasis to the lungs with UBS109: M. Shoji, et al.; Oncotarget 9, 36102 (2018)
- Curcumin and derivatives function through protein phosphatase 2A and presenilin orthologues in Dictyostelium discoideum: M. Cocorocchio, et al.; Dis. Mod. Mechan. 11, dmm032375 (2018)