Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
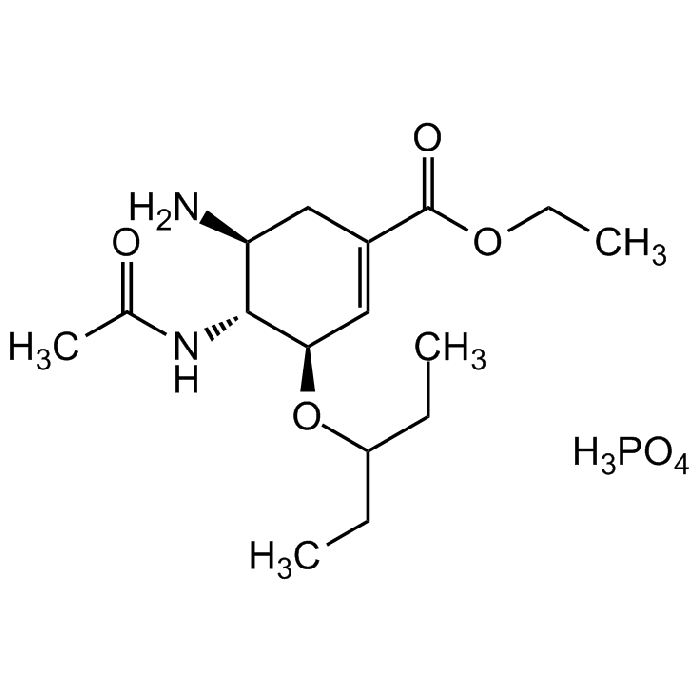
Oseltamivir . phosphate salt
As low as
35
CHF
CHF 35.00
In stock
Only %1 left
AG-CR1-3714-M02525 mgCHF 35.00
AG-CR1-3714-M100100 mgCHF 55.00
AG-CR1-3714-M250250 mgCHF 110.00
| Product Details | |
|---|---|
| Synonyms | GS-4104; Ro 64-0796/002; Tamiflu |
| Product Type | Chemical |
| Properties | |
| Formula |
C16H28N2O4 . H3PO4 |
| MW | 312.4 . 98.0 |
| CAS | 204255-11-8 |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white solid. |
| Solubility | Soluble in DMSO (10mg/ml) or water (10mg/ml). |
| Identity | Determined by 1H-NMR. |
| InChi Key | POPJIXMLXGJJOV-ZFSDTUNKSA-N |
| Smiles | O=P(OO)=O.N[C@@H]1[C@@H](NC(C)=O)[C@H](OC(CC)CC)C=C(C(OCC)=O)C1.[HH] |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Keep cool and dry. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Oseltamivir phosphate is an influenza viral neuraminidase inhibitor. It is an antiviral prodrug targeted against the influenza viruses. Once hydrolyzed in the liver to its active metabolite, oseltamivir carboxylate, it can competitively inhibit viral neuraminidase (IC50s=0.1-4.9nM for influenza neuraminidases A and B). The enzyme cleaves the sialic acid which is found on glycoproteins on the surface of human cells that helps new virions to exit the cell. Thus oseltamivir is blocking the release of new viral particles from a host cell.
- Oseltamivir phosphate is used clinically to treat influenza A and influenza B and to prevent flu after exposure. It potentially could be used to reduce the spread of the SARS-CoV-2 and counteract against the transmission of COVID-19.
Product References
- Inhibition of influenza virus infections in mice by GS4104, an orally effective influenza virus neuraminidase inhibitor: R.W. Sidwell, et al.; Antiviral Res. 37, 107 (1998)
- Use of the selective oral neuraminidase inhibitor oseltamivir to prevent influenza: F.G. Hayden, et al.; N. Engl. J. Med. 341, 1336 (1999)
- Identification of GS 4104 as an orally bioavailable prodrug of the influenza virus neuraminidase inhibitor GS 4071: W. Li, et al.; Antimicr. Agents Chemother. 42, 647 (1998)
- Biexponential decomposition of a neuraminidase inhibitor prodrug (GS-4104) in aqueous solution: R. Oliyai, et al.; Pharm. Res. 15, 1300 (1998)
- Carbocyclic influenza neuraminidase inhibitors possessing a C3-cyclic amine side chain: Synthesis and inhibitory activity: W. Lew, et al.; Bioorg. Med. Chem. Lett. 10, 1257 (2000)
- Discovery and development of GS 4104 (oseltamivir): an orally active influenza neuraminidase inhibitor: W. Lew, et al.; Curr. Med. Chem. 7, 663 (2000) (Review)
- Synthesis of potent pyrrolidine influenza neuraminidase inhibitors: A.C. Krueger, et al.; Bioorg. Med. Chem. Lett. 18, 1692 (2008)
- Susceptibility of highly pathogenic H5N1 influenza viruses to the neuraminidase inhibitor oseltamivir differs in vitro and in a mouse model: E.A. Govorkova, et al.; Antimicr. Agents Chemother. 53, 3088 (2009)
- Coronavirus puts drug repurposing on the fast track: C. Harrison; Nat. Biotech. NEWS (2020)