Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
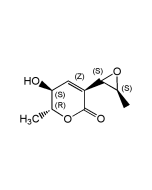
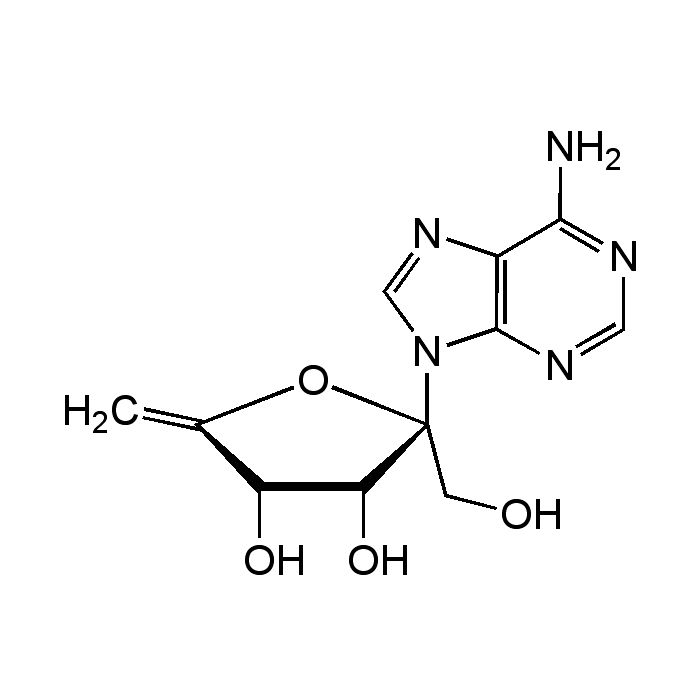
Decoyinine
As low as
85
CHF
CHF 85.00
In stock
Only %1 left
BVT-0030-M0011 mgCHF 85.00
BVT-0030-M0055 mgCHF 335.00
| Product Details | |
|---|---|
| Synonyms | Angustmycin A; U-7984; 9-(6-Deoxy-D-β-erythro-hex-5-en-2-ulofuranosyl)-adenine |
| Product Type | Chemical |
| Properties | |
| Formula |
C11H13N5O4 |
| MW | 279.3 |
| CAS | 2004-04-8 |
| RTECS | AU6256000 |
| Source/Host Chemicals | Isolated from Streptomyces sp. S 2113. |
| Purity Chemicals | ≥98% (NMR) |
| Appearance | White to off-white solid. |
| Solubility | Soluble in DMSO (warm), water or 100% ethanol: poorly soluble in methanol. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | UZSSGAOAYPICBZ-SOCHQFKDSA-N |
| Smiles | NC1=NC=NC2=C1N=CN2[C@]1(CO)OC(=C)C(O)[C@@H]1O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light when in solution. |
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Nucleoside antibiotic.
- Antitumor compound.
- Xanthosine monophosphate (XMP) aminase inhibitor.
- RNA synthesis inhibitor.
- Specific GMP synthase inhibitor.
- Reduces intracellular GTP levels.
Product References
- Studies on a new antibiotic, angustmycin: H. Sakai, et al.; J. Antibiot. 7, 116 (1954)
- Studies on a new antibiotic, angustmycin. I: H. Yuntsen, et al.; J. Antibiot. 7, 113 (1954)
- Studies on angustmycin. III: H. Yuntsen, et al.; J. Antibiot. 9, 195 (1956)
- On the studies of angustmycins. VI. Chemical structure of angustmycin A: H. Yuntsen; J. Antibiot. 11, 79 (1958)
- Purine nucleosides. XXII. The synthesis of angustmycin A (decoyinine) and related unsaturated nucleosides: J.R. McCarthy, Jr., et al.; J. Am. Chem. Soc. 90, 4993 (1968)
- Effects of several tumor-inhibitory antibiotics on immunological responses: H. Yamaki, et al.; J. Antibiot. 22, 315 (1969)
- Halo sugar nucleosides. 5. Synthesis of angustmycin A and some base analogues: E.J. Prisbe, et al.; J. Org. Chem. 41, 1836 (1976)
- A decrease in GTP content is associated with aerial mycelium formation in Streptomyces MA406-A-1: K. Ochi; J. Gen. Microbiol. 132, 299 (1986)
- Extracellular control of spore formation in Bacillus subtilis: A.D. Grossman & R. Losick; PNAS 85, 4369 (1988)
- Nucleoside antibiotics: structure, biological activity, and biosynthesis: K. Isono; J. Antibiot. 41, 1711 (1988)
- A target for carbon source-dependent negative regulation of the citB promoter of Bacillus subtilis: A. Fouret & A.L. Sonenshein; J. Bacteriol. 172, 835 (1990)
- Sporulation of Streptomyces venezuelae in submerged cultures: M.A. Glazebrook, et al.; J. Gen. Microbiol. 136, 581 (1990)
- Conformational studies on some Cl'-branched beta-D-nucleosides by 1H-NMR spectroscopy and molecular mechanics calculations: J. Plavec, et al.; J. Biochem. Biophys. Meth. 26, 317 (1993)
- Biochemical characterization of human GMP synthetase: J. Nakamura & L. Lou; J. Biol. Chem. 270, 7347 (1995)
- Guanine nucleotides guanosine 5'-diphosphate 3'-diphosphate and GTP co-operatively regulate the production of an antibiotic bacilysin in Bacillus subtilis: T. Inaoka, et al.; J. Biol. Chem. 278, 2169 (2003)
- Expression of kinA and kinB of Bacillus subtilis, necessary for sporulation initiation, is under positive stringent transcription control: S. Tojo, et al.; J. Bacteriol. 195, 1656 (2013)
- Pharmacological targeting of guanosine monophosphate synthase suppresses melanoma cell invasion and tumorigenicity: A. Bianchi-Smiraglia, et al.; Cell Death Differ. 22, 1858 (2015)