Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
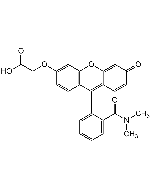
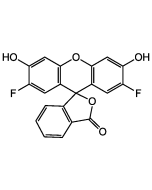
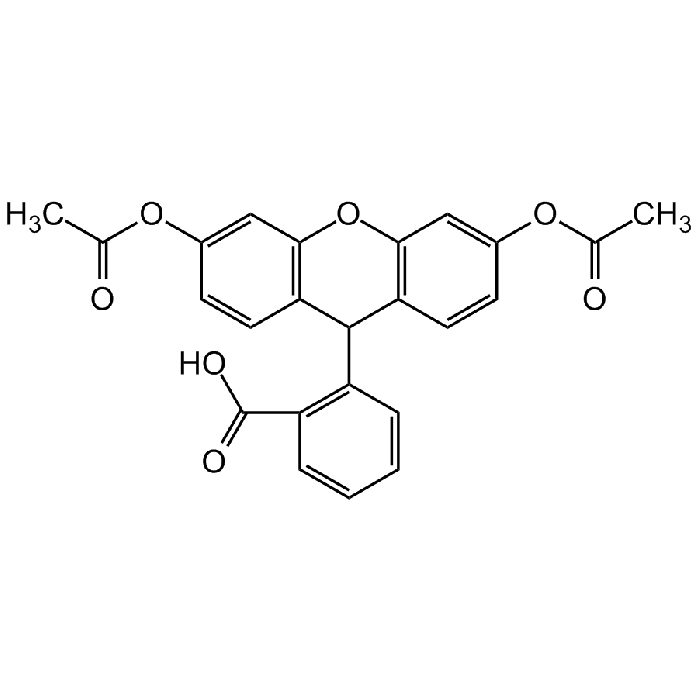
Dihydrofluorescein diacetate
| Product Details | |
|---|---|
| Product Type | Chemical |
| Properties | |
| Formula | C24H18O7 |
| MW | 418.4 |
| CAS | 35340-49-9 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥97% (NMR) |
| Appearance | Solid. |
| Solubility | Soluble in chloroform. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | YKSJJXGQHSESKB-UHFFFAOYSA-N |
| Smiles | CC(=O)OC1=CC2=C(C=C1)C(C1=C(O2)C=C(OC(C)=O)C=C1)C1=C(C=CC=C1)C(O)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Dihydrofluorescein diacetate is a fluorescent probe for detecting intracellular oxidants. It is hydrolyzed by cellular esterases to dihydrofluorescein and is then oxidized to fluorescein primarily by H2O2. Dihydrofluorescein diacetate might be reactive toward a broad range of oxidizing reactions that may be increased during intracellular oxidant stress. Cell-loading studies indicate that dihydrofluorescein achieves higher intracellular concentrations than the other redox sensors such as Dihydrorhodamine 123 (Ex/Em: 490/514nm).
(1) P.P. Williams & R.L. Stolzenberg; Applied Microbiology 23(4), 745-9 (1972) | (2) S.L. Hempel, et al.; Free Radical Biology & Medicine 27(1/2), 146-159 (1999) | (3) Y. O'Malley et al.; Free Radical Biology & Medicine 36(1), 90-100 (2004)