Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
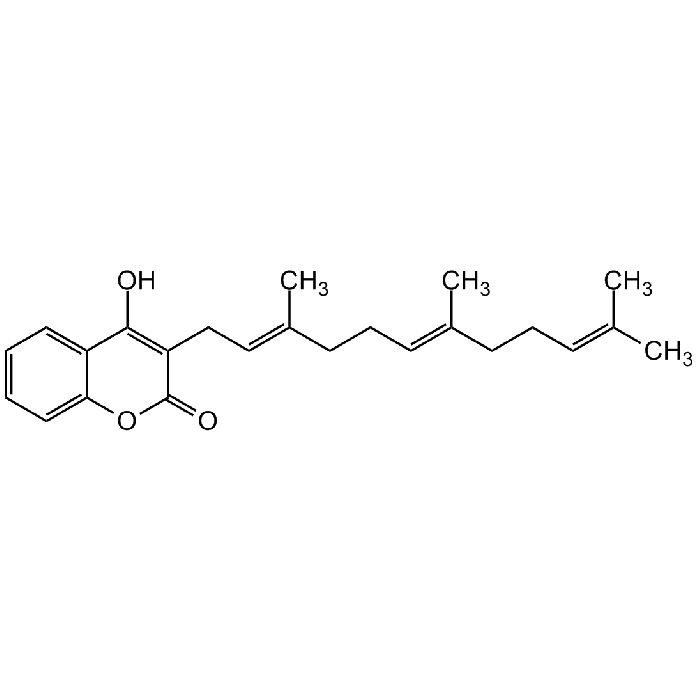
Ferulenol
As low as
50
CHF
CHF 50.00
In stock
Only %1 left
AG-CN2-0011-M0011 mgCHF 50.00
AG-CN2-0011-M0055 mgCHF 200.00
AG-CN2-0011-M01010 mgCHF 340.00
| Product Details | |
|---|---|
| Synonyms | 4-Hydroxy-3-((2E,6E)-3,7,11-trimethyldodeca-2,6,10-trien-1-yl)-2H-chromen-2-one |
| Product Type | Chemical |
| Properties | |
| Formula |
C24H30O3 |
| MW | 366.5 |
| CAS | 6805-34-1 |
| Source/Host Chemicals | Isolated from Ferula communis. |
| Purity Chemicals | ≥97% (HPLC) |
| Appearance | White to off-white solid. |
| Solubility | Soluble in 100% ethanol, methanol or DMSO. |
| Identity | Determined by 1H-NMR. |
| InChi Key | NJJDBBUWWOAOLD-CFBAGHHKSA-N |
| Smiles | OC(C1=CC=CC=C1O2)=C(C/C=C(C)/CC/C=C(C)/CC/C=C(C)/C)C2=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Protect from light. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Prenylated 4-hydroxycoumarin.
- Anti-tumor compound [2].
- Cytotoxic [2].
- Stimulator of tubulin polymerization in vitro [2].
- Inhibitor of colchicine binding to tubulin [2].
- Antitubercular antibiotic with potent antibacterial activity [3].
- Anti-coagulant, pro-haemorrhagic compound with higher activity than warfarin [4].
- Shows hepatocyte toxicity [1, 4].
- Disrupts mitochondrial membrane potential [5, 6].
- Potent L-malate:quinone oxidoreductase (PfMQO) inhibitor in Plasmodium falciparum.
- Antimalarial.
Product References
- Acute toxicity of ferulenol, a 4-hydroxycoumarin isolated from Ferula communis L: O. Fraigui, et al.; Vet. Hum. Toxicol. 44, 5 (2002)
- Microtubule-interacting activity and cytotoxicity of the prenylated coumarin ferulenol: C. Bocca, et al.; Planta Med. 68, 1135 (2002)
- Antimycobacterial coumarins from the sardinian giant fennel (Ferula communis): G. Appendino, et al.; J. Nat. Prod. 67, 210 (2004)
- Characterization of anti-coagulant properties of prenylated coumarin ferulenol: M. Monti, et al.; Biochim. Biophys. Acta 1770, 1437 (2007)
- Ferulenol specifically inhibits succinate ubiquinone reductase at the level of the ubiquinone cycle: M. Lahouel, et al.; BBRC 355, 252 (2007)
- Disruption of mitochondrial membrane potential by ferulenol and restoration by propolis extract: antiapoptotic role of propolis: B.H. Nadia, et al.; Acta Biol. Hung. 60, 385 (2009)
- Biochemical studies of membrane bound Plasmodium falciparum mitochondrial L-malate:quinone oxidoreductase, a potential drug target: E.D. Hartuti, et al.; BBA Bioenerg. 1859, 191 (2018)
- Plasmodium Parasite Malate-Quinone Oxidoreductase Functionally Complements a Yeast Deletion Mutant of Mitochondrial Malate Dehydrogenase: T. Ito, et al.; Microbiol. Spectr. ahead of print (2023)