Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
Etoposide
As low as
30
CHF
CHF 30.00
In stock
Only %1 left
AG-CR1-3572-M02525 mgCHF 30.00
AG-CR1-3572-M100100 mgCHF 70.00
AG-CR1-3572-51005 x 100 mgCHF 280.00
| Product Details | |
|---|---|
| Synonyms | VP-16-213; NSC 141540 |
| Product Type | Chemical |
| Properties | |
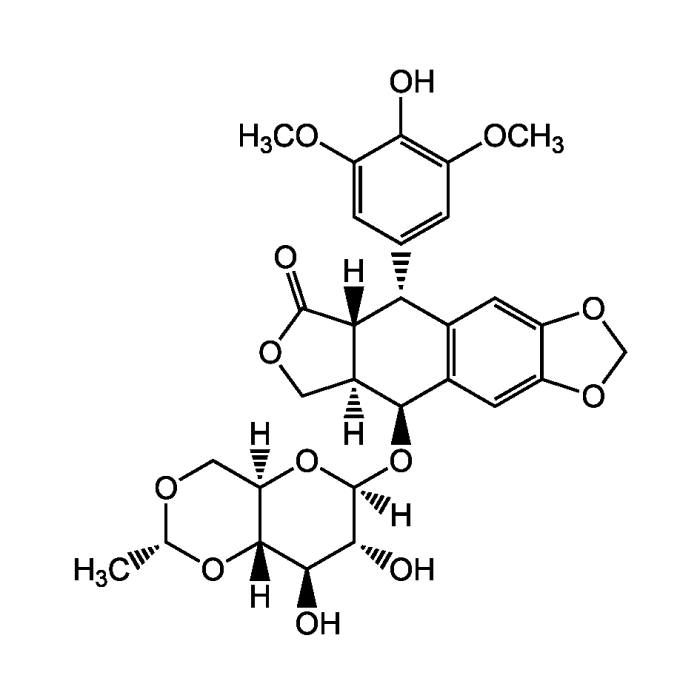
| Formula |
C29H32O13 |
| MW | 588.6 |
| Merck Index | 14: 3886 |
| CAS | 33419-42-0 |
| RTECS | KC0190000 |
| Source/Host Chemicals | Semisynthetic derivative of podophyllotoxin. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO, ethanol, methanol or dimethylformamide. |
| InChi Key | VJJPUSNTGOMMGY-MRVIYFEKSA-N |
| Smiles | [H][C@]12COC(=O)[C@]1([H])[C@H](C1=CC(OC)=C(O)C(OC)=C1)C1=C(C=C3OCOC3=C1)[C@H]2O[C@]1([H])O[C@]2([H])CO[C@@H](C)O[C@@]2([H])[C@H](O)[C@H]1O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability |
Stable for at least 2 years after receipt when stored at +20°C. Store solutions in DMSO at 4°C. After reconstitution, prepare aliquots and store at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Potent anti-cancer compound. Induces apoptosis in normal and tumor cell lines [1, 5].
- DNA Topoisomerase II activity inhibitor. Increases Topo II-mediated DNA breakage primarily by inhibiting the ability of the enzyme to religate cleaved nucleic acid molecules. Does not lead to immediate block of DNS synthesis, induces a progressive inhibition of DNA replication [2, 4, 6].
- p53 activator [3].
- Blocks the cell cycle between the end of the S phase and the early G2 phase [2, 8].
- Oncoprotein Mdm2 synthesis inhibitor [7].
- Apoptosis inducer through the cytochrome c/Apaf-1/caspase-9 pathway and the Fas-mediated death signaling pathway [9, 10].
- Cell cycle checkpoint activator. Affects gene expression at different levels (chromatin remodeling, transcription and alternative splicing) [11, 14, 15].
- Chemotherapeutic compound used in cancers [12, 13].
- Used in conditioning regimen prior to a bone marrow or blood stem cell transplantation [16].
- Highly effective in mobilizing stem cells [17]
Product References
- The podophyllotoxin derivatives VP16-213 and VM26: B.F. Issell; Cancer Chemother. Pharmacol. 7, 73 (1982)
- Topoisomerase-specific drug sensitivity in relation to cell cycle progression: K.C. Chow & W.E. Ross; Mol. Cell. Biol. 7, 3119 (1987)
- Increases in sequence specific DNA binding by p53 following treatment with chemotherapeutic and DNA damaging agents: R.B. Tishler, et al.; Cancer Res. 53, 2212 (1993)
- Topoisomerase II-etoposide interactions direct the formation of drug-induced enzyme-DNA cleavage complexes: D.A. Burden, et al.; J. Biol. Chem. 271, 29238 (1996)
- Cell death induced by topoisomerase-targeted drugs: more questions than answers: S.H. Kaufmann; Biochim. Biophys. Acta 1400, 195 (1998) (Review)
- Etoposide: four decades of development of a topoisomerase II inhibitor: K.R. Hande; Eur. J. Cancer 34, 1514 (1998) (Review)
- Differential regulation of p21waf-1/cip-1 and Mdm2 by etoposide: etoposide inhibits the p53-Mdm2 autoregulatory feedback loop: E.L. Arriola, et al.; Oncogene 18, 1081 (1999)
- Early caspase activation in leukemic cells subject to etoposide-induced G2-M arrest: evidence of commitment to apoptosis rather than mitotic cell death: R.J. Sleiman & B.W. Stewart; Clin. Cancer Res. 6, 3756 (2000)
- Ordering of ceramide formation, caspase activation, and Bax/Bcl-2 expression during etoposide-induced apoptosis in C6 glioma cells: M. Sawada, et al.; Cell Death Differ. 7, 761 (2000)
- Distinct pathways for stimulation of cytochrome c release by etoposide: J.D. Robertson, et al.; J. Biol. Chem. 275, 32438 (2000)
- Deacetylase activity associates with topoisomerase II and is necessary for etoposide-induced apoptosis: C.A. Johnson, et al.; J. Biol. Chem. 276, 4539 (2001)
- Etoposide: discovery and medicinal chemistry: P. Meresse, et al.; Curr. Med. Chem. 11, 2443 (2004) (Review)
- Etoposide, topoisomerase II and cancer: E.L. Baldwin & N. Osheroff; Curr. Med. Chem. Anticancer Agents 5, 363 (2005) (Review)
- The dispersal of replication proteins after Etoposide treatment requires the cooperation of Nbs1 with the ataxia telangiectasia Rad3-related/Chk1 pathway: R. Rossi, et al.; Cancer Res. 66, 1675 (2006)
- Cellular response to etoposide treatment: A. Montecucco & G. Biamonti; Cancer Lett. 252, 9 (2007) (Review)
- Chemomobilization with Etoposide is Highly Effective in Patients with Multiple Myeloma and Overcomes the Effects of Age and Prior Therapy: W.A. Wood, et al.; Biol. Blood Marrow. Transplant. 17, 141 (2011)
- The aging effect of chemotherapy on cultured human mesenchymal stem cells. S. Buttiglieri, et al.; Exp. Hematol. 39, 1171 (2011)