Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
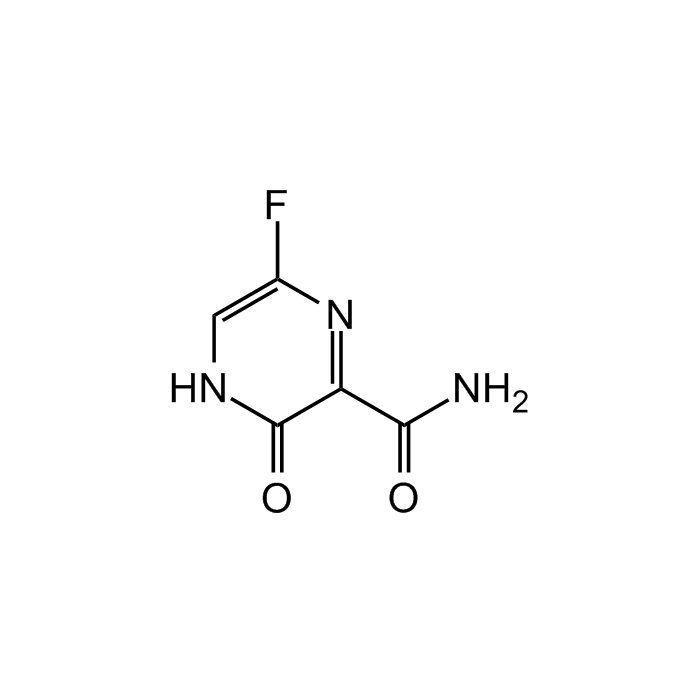
Favipiravir
| Product Details | |
|---|---|
| Synonyms | 6-Fluoro-3,4-dihydro-3-oxo-2-pyrazinecarboxamide; Favilavir; T-705; Avigan |
| Product Type | Chemical |
| Properties | |
| Formula |
C5H4FN3O2 |
| MW | 157.1 |
| CAS | 259793-96-9 |
| Purity Chemicals | ≥98% |
| Appearance | White to off-white solid. |
| Solubility | Soluble in DMSO (30mg/ml) or DMF (25mg/ml). |
| InChi Key | ZCGNOVWYSGBHAU-UHFFFAOYSA-N |
| Smiles | FC1=CNC(C(C(N)=O)=N1)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Keep cool and dry. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
-
The guanine analog Favipiravir is a broad spectrum inhibitor of viral RNA polymerase, acting on viral genetic copying to prevent its reproduction.
-
Favipiravir is a prodrug that is metabolized to its active form, favipiravir-ribofuranosyl-5'-triphosphate (Favipiravir-RTP), which inhibits selectively RNA-dependent RNA polymerase (RdRp) in RNA viruses.
-
This antiviral agent inhibits influenza A, B and C without inducing cytotoxicity up to 1,000 µg/ml. It also inhibits influenza strains resistant to current antiviral drugs and shows a synergistic effect in combination with oseltamivir (Prod. No. AG-CR1-3714), thereby expanding influenza treatment options.
-
Favipiravir is active against a variety of additional viruses, including West Nile virus, yellow fever virus, foot-and-mouth disease virus as well as other flaviviruses, arenaviruses, bunyaviruses and alphaviruses in vitro and in vivo.
-
Activity has also been shown against enteroviruses, Rift Valley fever virus and Marburg virus (MARV9), while limited efficacy has been shown against Ebola virus (EBOV) and Zika virus in animal studies.
-
Favipiravir shows potential efficacy against SARS-CoV-2 and is undergoing clinical studies to test its efficacy and safety in the treatment of coronavirus disease 2019 (COVID-19).
- In vitro and in vivo activities of anti-influenza virus compound T-705: Y. Furuta, et al.; Antimicrob. Agents Chemother. 46, 977 (2002)
- Mechanism of action of T-705 against influenza virus: Y. Furuta, et al.; Antimicrob. Agents Chemother. 49, 981 (2005)
- Efficacy of orally administered T-705 on lethal avian influenza A (H5N1) virus infections in mice: R.W. Sidwell, et al.; Antimicrob. Agents Chemother. 51, 845 (2007)
- Activity of T-1106 in a hamster model of yellow Fever virus infection: J.G. Julander, et al.; Antimicrob. Agents Chemother. 51, 1962 (2007)
- In vitro and in vivo activities of T-705 against arenavirus and bunyavirus infections: B.B. Gowen, et al.; Antimicrob. Agents Chemother. 51, 3168 (2007)
- Efficacy of orally administered T-705 pyrazine analog on lethal West Nile virus infection in rodents: J.D. Morrey, et al.; Antiviral Res. 80, 377 (2008)
- Favipiravir (T-705) inhibits in vitro norovirus replication: J. Rocha-Pereira, et al.; BBRC 424, 777 (2012)
- Role of human hypoxanthine guanine phosphoribosyltransferase in activation of the antiviral agent T-705 (favipiravir): L. Naesens, et al.; Mol. Pharmacol. 84, 615 (2013)
- Mechanism of action of T-705 ribosyl triphosphate against influenza virus RNA polymerase: H. Sangawa, et al.; Antimicrob. Agents Chemother. 57, 5202 (2013)
- Favipiravir (T-705), a novel viral RNA polymerase inhibitor: Y. Furuta, et al.; Antiviral Res. 100, 446 (2013) (Review)
- T-705 (Favipiravir) suppresses tumor necrosis factor α production in response to influenza virus infection: A beneficial feature of T-705 as an anti-influenza drug: T. Tanaka, et al.; Acta Virol. 61, 48 (2017)Antiviral activity of favipiravir (T-705) against mammalian and avian bornaviruses: T. Tokunaga, et al.; Antiviral Res. 143, 237 (2017)
- Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase: Y. Furuta, et al.; Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 93, 449 (2017) (Review)
- Zika Virus Replication Is Substantially Inhibited by Novel Favipiravir and Interferon Alpha Combination Regimens: C.P. Pires de Mello, et al.; Antimicrob. Agents Chemother. 62, e01983 (2017)
- Efficacy of favipiravir (T-705) in nonhuman primates infected with Ebola virus or Marburg virus: S.L. Bixler, et al.; Antiviral Res. 151, 97 (2018)
- Favipiravir, an anti-influenza drug against life-threatening RNA virus infections: K. Shiraki & T. Daikoku; Pharmacol. Ther. (Epub ahead of print) (2020) (Review)
- Discovering drugs to treat coronavirus disease 2019 (COVID-19): L. Dong, et al.; Drug Discov. Ther. 14, 58 (2020)