Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
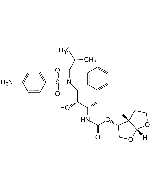
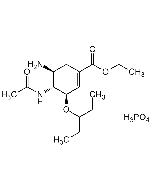
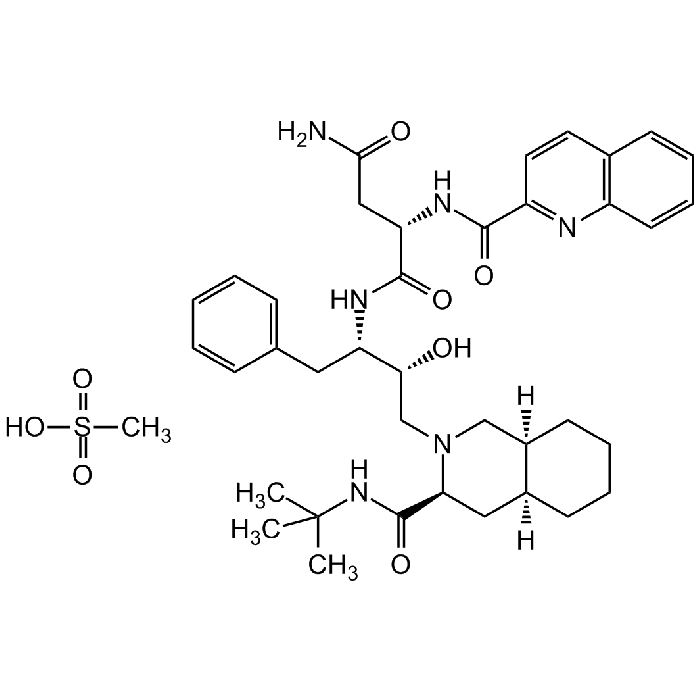
Saquinavir . mesylate
As low as
70
CHF
CHF 70.00
In stock
Only %1 left
AG-CR1-3727-M01010 mgCHF 70.00
AG-CR1-3727-M05050 mgCHF 210.00
| Product Details | |
|---|---|
| Synonyms | Ro 31-8959 |
| Product Type | Chemical |
| Properties | |
| Formula |
C38H50N6O5 . CH4SO3 |
| MW | 670.9 . 96.1 |
| CAS | 149845-06-7 |
| RTECS | EJ6664000 |
| Purity Chemicals | ≥98% |
| Appearance | White to off-white solid. |
| Solubility | Soluble in DMSO (10mg/ml). |
| Identity | Determined by 1H-NMR. |
| InChi Key | IRHXGOXEBNJUSN-YOXDLBRISA-N |
| Smiles | CS(=O)(O)=O.O[C@@H]([C@@H](NC([C@H](CC(N)=O)NC(C1=NC(C=CC=C2)=C2C=C1)=O)=O)CC3=CC=CC=C3)CN4C[C@@]5([H])CCCC[C@@]5([H])C[C@H]4C(NC(C)(C)C)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Keep cool and dry. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Saquinavir is an antiretroviral inhibitor of the human immunodeficiency virus. It potently inhibits HIV-1 (Ki=0.12nM) and HIV-2 (Ki<0.1nM) protease in vitro. HIV-1 protease is important for the replication of the virus within the cell and the release of mature viral particles from the infected cell.
- Formulations containing saquinavir have been used in combination with other inhibitors for the treatment of AIDS.
- Saquinavir shows other antimicrobial and antiviral activity. Potential SARS-CoV-2 replication inhibitor, responsible for COVID-19.
- Shows anti-cancer activity. Shown to induce apoptosis, autophagy and endoplasmic reticulum stress, which consequently leads to cancer cell death. Inhibits chymotrypsin-like and caspase-like activity of the 26S proteasome and purified 20S proteasome.
- Anti-inflammatory agent. Blocks cathepsin V and thereby inhibits disulfide HMGB1-induced TLR4 activation and cytokine production.
Product References
- Rational design of peptide-based HIV proteinase inhibitors: N.A. Roberts, et al.; Science 248, 358 (1990)
- A series of potent HIV-1 protease inhibitors containing a hydroxyethyl secondary amine transition state isostere: Synthesis, enzyme inhibition, and antiviral activity: T.J. Tucker, et al.; J. Med. Chem. 35, 2525 (1992)
- Saquinavir: an HIV proteinase inhibitor: K. Bragman; Adv. Exp. Med. Biol. 394, 305 (1996)
- The human immunodeficiency virus (HIV)-1 protease inhibitor saquinavir inhibits proteasome function and causes apoptosis and radiosensitization in non-HIV-associated human cancer cells: F. Pajonk, et al.; Cancer Res. 62, 5230 (2002)
- The HIV protease inhibitors saquinavir, ritonavir, and nelfinavir induce apoptosis and decrease barrier function in human intestinal epithelial cells: H. Bode, et al.; Antivir. Ther. 10, 645 (2005)
- The HIV protease inhibitor saquinavir induces endoplasmic reticulum stress, autophagy, and apoptosis in ovarian cancer cells: K. McLean, et al.; Gynecol. Oncol. 112, 623 (2009)
- Saquinavir, the pioneer antiretroviral protease inhibitor: C.J. la Porte; Expert Opin. Drug Metab. Toxicol. 5, 1313 (2009) (Review)
- Saquinavir inhibits the malaria parasite's chloroquine resistance transporter: R.E. Martin, et al.; Antimicrob. Agents Chemother. 56, 2283 (2012)
- The antiretroviral agent saquinavir enhances hTERT expression and telomerase activity in human T leukaemia cells in vitro: R. Adamo, et al.; J. Exp. Clin. Cancer Res. 32, 38 (2013)
- The HIV Protease Inhibitor Saquinavir Inhibits HMGB1-Driven Inflammation by Targeting the Interaction of Cathepsin V with TLR4/MyD88: J.P. Pribis, et al.; Mol. Med. 21, 749 (2015)
- Saquinavir Ameliorates Liver Warm Ischemia-Reperfusion-Induced Lung Injury via HMGB-1- and P38/JNK-Mediated TLR-4-Dependent Signaling Pathways: Z. Yu, et al.; Mediators Inflamm. 2017, 7083528 (2017)
- A search for medications to treat COVID-19 via in silico molecular docking models of the SARS-CoV-2 spike glycoprotein and 3CL protease: D.C. Hall & H.F. Ji; Travel Med Infect Dis. (Epub ahead of print) (2020)
- Identification of chymotrypsin-like protease inhibitors of SARS-CoV-2 via integrated computational approach: S.A. Khan, et al.; J. Biomol. Struct. Dyn. 39, 2607 (2021)
- Unrevealing sequence and structural features of novel coronavirus using in silico approaches: The main protease as molecular target: J.T. Ortega, et al.; EXCLI J. 19, 400 (2020)
- Identification of FDA Approved Drugs Targeting COVID-19 Virus by Structure-Based Drug Repositioning: P. Wang, et al.; ChemRxiv (Preprint) (2020)