Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
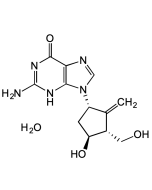
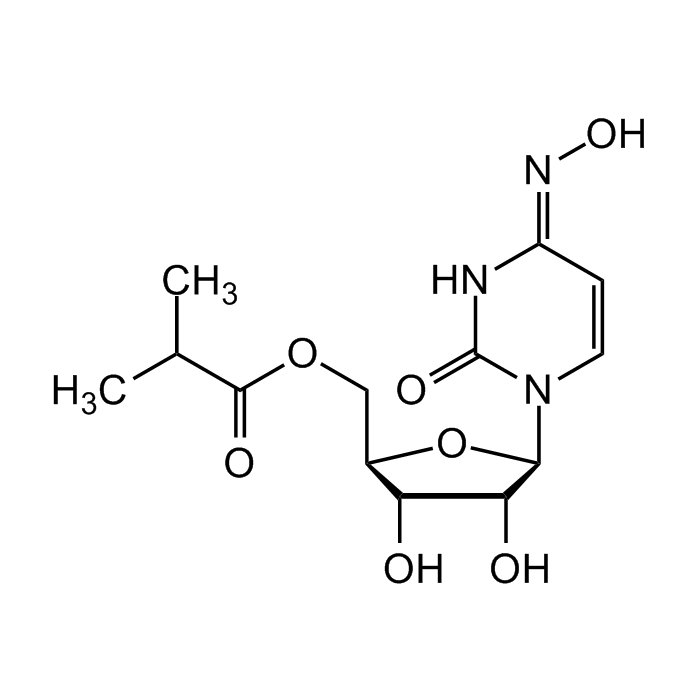
EIDD-2801
As low as
80
CHF
CHF 80.00
In stock
Only %1 left
AG-CR1-3733-M01010 mgCHF 80.00
AG-CR1-3733-M05050 mgCHF 200.00
| Product Details | |
|---|---|
| Synonyms | β-D-N4-Hydroxycytidine-5'-isopropyl ester; EIDD 2801; MK-4482; Molnupiravir |
| Product Type | Chemical |
| Properties | |
| Formula |
C13H19N3O7 |
| MW | 329.3 |
| CAS | 2349386-89-4 |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white solid. |
| Solubility | Soluble in DMSO (25mg/ml), DMF (25mg/ml) or water (2mg/ml). |
| Identity | Determined by 1H-NMR. |
| InChi Key | HTNPEHXGEKVIHG-QCNRFFRDSA-N |
| Smiles | O=C(N(C=C/1)[C@@H]2O[C@H](COC(C(C)C)=O)[C@@H](O)[C@H]2O)NC1=N\O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Keep cool and dry. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- EIDD-2801 is an orally bioavailable prodrug of the antiviral nucleoside derivative N4-hydroxycytidine (NHC, EIDD-1931).
- It is a nucleotide analog inhibitor of RNA-dependent RNA polymerases (RdRps). The compound interferes with the action of viral RNA polymerase. It exerts its antiviral action through introduction of copying errors during viral RNA replication. The active drug incorporates into the genome of RNA viruses, leading to an accumulation of mutations known as viral error catastrophe. This mechanism of inhibition of viral replication is also known as lethal mutagenesis.
- The broad-spectrum antiviral agent EIDD-2801 inhibits viral RNA replication in various unrelated RNA viruses including influenza, Ebola, Venezuelan equine encephalitis virus (VEEV) and coronaviruses, including SARS-CoV, MERS-CoV and SARS-CoV-2.
- EIDD-2801 has the potential for COVID-19, seasonal and pandemic influenza treatment.
- Treatment of SARS-CoV-2 infection with EIDD-2801 completely suppressed virus transmission within 24 hours in ferrets.
Product References
- Characterization of orally efficacious influenza drug with high resistance barrier in ferrets and human airway epithelia: M.Toots, et al.; Sci. Transl. Med. 11, 515 (2019)
- New Flu Antiviral Candidate May Thwart Drug Resistance: T. Hampton; JAMA 323, 17 (2020)
- Quantitative efficacy paradigms of the influenza clinical drug candidate EIDD-2801 in the ferret model: M. Toots, et al.; Transl. Res. 218, 16 (2020)
- An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice: T.P. Sheahan, et al.; Sci. Transl. Med. 12, 541 (2020)
- Orally delivered MK-4482 inhibits SARS-CoV-2 replication in the Syrian hamster model: K. Rosenke, et al.; Nat. Commun. 12, 2295 (2021)
- Therapeutically administered ribonucleoside analogue MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets: R.M. Cox, et al.; Nat. Microbiol. 6, 11 (2021)
- Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis: F. Kabinger, et al.; Nat. Struct. Mol. Biol. 28, 740 (2021)
- Molnupiravir Inhibits Replication of the Emerging SARS-CoV-2 Variants of Concern in a Hamster Infection Model: R. Abdelnabi, et al.; J. Infect. Dis. 224, 749 (2021)
- Accelerated first-in-human clinical trial of EIDD-2801/MK-4482 (molnupiravir), a ribonucleoside analog with potent antiviral activity against SARS-CoV-2: W. Holman, et al.; Trials 22, 561 (2021)
- Developing a direct acting, orally available antiviral agent in a pandemic: the evolution of molnupiravir as a potential treatment for COVID-19: G.R. Painter, et al.; Curr. Opin. Virol. 50, 17 (2021)
- Molnupiravir: coding for catastrophe; B. Malone & E.A. Campbell; Nat. Struct. Mol. Biol. 28, 706 (2021)