Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
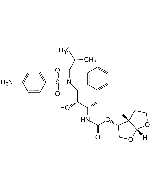
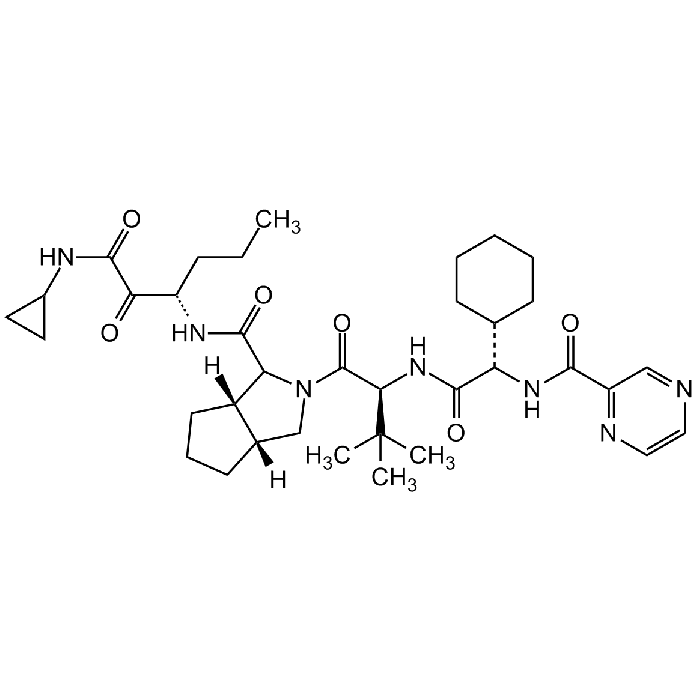
Telaprevir
As low as
100
CHF
CHF 100.00
In stock
Only %1 left
AG-CR1-3741-M01010 mgCHF 100.00
AG-CR1-3741-M05050 mgCHF 300.00
AG-CR1-3741-M250250 mgCHF 620.00
| Product Details | |
|---|---|
| Synonyms | VX-950; LY570310; MP-424 |
| Product Type | Chemical |
| Properties | |
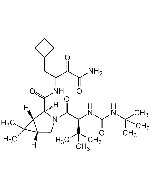
| Formula |
C36H53N7O6 |
| MW | 679.9 |
| CAS | 402957-28-2 |
| Purity Chemicals | ≥95% (HPLC) |
| Appearance | White to off-white solid. |
| Solubility | Soluble in DMSO (10mg/ml) or DMF. |
| Identity | Determined by 1H-NMR. |
| InChi Key | BBAWEDCPNXPBQM-DXXZLHHHSA-N |
| Smiles | O=C([C@H](C(C)(C)C)NC([C@H](C1CCCCC1)NC(C2=NC=CN=C2)=O)=O)N3C(C(N[C@@H](CCC)C(C(NC4CC4)=O)=O)=O)[C@](CCC5)([H])[C@]5([H])C3 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice | Keep cool and dry. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- The antiviral agent telaprevir is a covalent, reversible inhibitor of hepatitis C virus (HCV) nonstructural protease 3/4A (NS3/4A; Ki = 7nM), a serine protease that is essential for viral replication by cleaving the virally encoded polyprotein into mature proteins like NS4A, NS4B, NS5A and NS5B, leading to inhibition of viral polyprotein processing and subsequently decrease of viral RNA replication. It inhibits viral replication in genotype 1b HCV replicon cells (IC50 = 354nM) and isolated human fetal hepatocytes infected with genotype 1a HCV-positive patient sera (IC50 = 280nM).
- Used in stabilizable polypeptide linkages (StaPLs) chemogenetic platform, for pharmacological regulation of protein expression/function. StaPL sequences can be engineered into target proteins rendering them functional only in the presence of telaprevir.
- Telaprevir has been identified as a potential inhibitor of SARS-CoV-2, responsible for COVID-19. It inhibits SARS-CoV-2 viral replication by targeting the viral 3C-like Protease (3CLpro), interacting through hydrogen bond formation with Ser46 and Gln182 main chain amines as well as Glu166 α-carboxyl group.
Product References
- Preclinical profile of VX-950, a potent, selective, and orally bioavailable inhibitor of hepatitis C virus NS3-4A serine protease: R.B. Perni, et al.; Antimicrob. Agents Chemother. 50, 899 (2006)
- Discovery and development of VX-950, a novel, covalent, and reversible inhibitor of hepatitis C virus NS3.4A serine protease: C. Lin, et al.; Infect. Disord. Drug Targets 6, 3 (2006) (Review)
- VX-950, a novel hepatitis C virus (HCV) NS3-4A protease inhibitor, exhibits potent antiviral activities in HCv replicon cells: K. Lin, et al.; Antimicrob. Agents Chemother. 50, 1813 (2006)
- Antiviral activity of telaprevir (VX-950) and peginterferon alfa-2a in patients with hepatitis C: N. Forestier, et al.; Hepatology 46, 640 (2007)
- Telaprevir: an NS3/4A protease inhibitor for the treatment of chronic hepatitis C: L.S. Smith, et al.; Ann. Pharmacother. 45, 639 (2011)
- Telaprevir: a novel NS3/4 protease inhibitor for the treatment of hepatitis C: O.M. Klibanov, et al.; Pharmacotherapy 31, 951 (2011) (Review)
- Discovery and development of telaprevir: an NS3-4A protease inhibitor for treating genotype 1 chronic hepatitis C virus: A.D. Kwong, et al.; Nat. Biotechnol. 29, 993 (2011)
- Telaprevir: a hepatitis C NS3/4A protease inhibitor: S.J. Matthews & J.W. Lancaster; Clin. Ther. 34, 1857 (2012)
- Telaprevir is a substrate and moderate inhibitor of P-glycoprotein, a strong inductor of ABCG2, but not an activator of PXR in vitro: J. Weiss, et al.; Int. J. Antimicrob. Agents. 43, 184 (2014)
- StaPLs: Versatile Genetically Encoded Modules for Engineering Drug-Inducible Proteins: C.L. Jacobs, et al.; Nat. Meth. 15, 523 (2018)
- In silico Repositioning for Dual Inhibitor Discovery of SARS-CoV-2 (COVID-19) 3C-like Protease and Papain-like Peptidase: K. Bagherzadeh, et al.; (Preprints) (2020)