Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
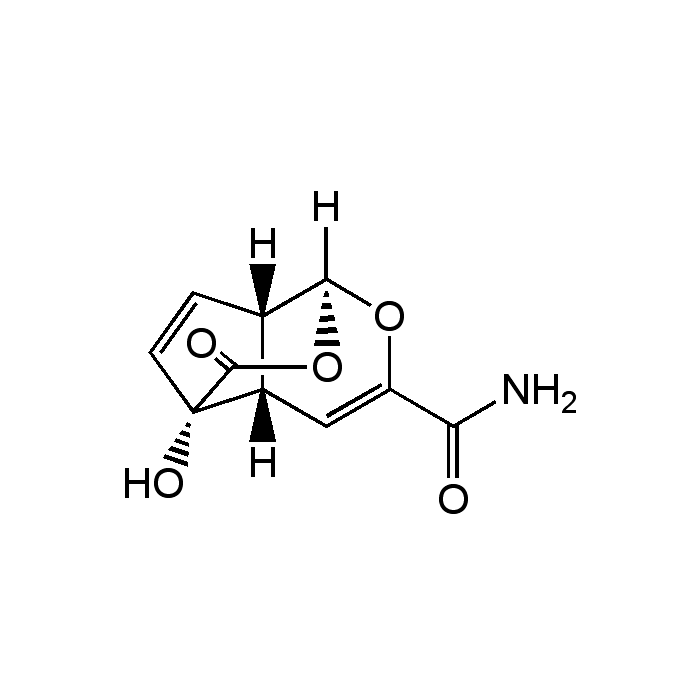
Echinosporin
As low as
155
CHF
CHF 155.00
In stock
Only %1 left
BVT-0006-M0011 mgCHF 155.00
BVT-0006-M0055 mgCHF 550.00
| Product Details | |
|---|---|
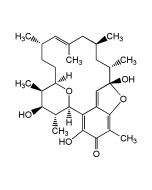
| Synonyms | NSC357683; XK 213; (1S,4aS,5S,7aS)-5-Hydroxy-8-oxo-1,4a,5,7a-tetrahydro-1,5-(epoxymethano)cyclopenta[c] pyran-3-carboxamide |
| Product Type | Chemical |
| Properties | |
| Formula |
C10H9NO5 |
| MW | 223.2 |
| CAS | 79127-35-8 |
| RTECS | JW5313000 |
| Source/Host Chemicals | Isolated from Streptomyces sp. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Off-white to beige solid. |
| Solubility | Soluble in water, methanol or dimethylformamide. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | OXSZHYWOGQJUST-PDXIVQBHSA-N |
| Smiles | [H][C@@]12C=C[C@@]3(O)C(=O)O[C@]1([H])OC(=C[C@@]23[H])C(N)=O |
| Shipping and Handling | |
| Shipping | BLUE ICE |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. Stock solutions are stable for at least 3 months when stored at -20°C. After reconstitution protect from light at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Antibiotic.
- Cell cycle inhibitor at the G(2)/M phase.
- Antitumor compound.
- Apoptosis inducer.
Product References
- A new antibiotic echinosporin (XK-213) - producing organism, isolation and characterization: T. Sato, et al.; J. Antibiot. 35, 266 (1982)
- Antitumor activity of echinosporin: M. Morimoto and R. Imai; J. Antibiot. 38, 490 (1985)
- Approaches to the total synthesis of the antitumor antibiotic echinosporin: M. A. Kinsella; J. Org. Chem. 55, 105 (1990)
- Applications of an asymmetric [2 + 2]-photocycloaddition. Total synthesis of (-)-echinosporin. Construction of an advanced 11-deoxyprostaglandin intermediate: A. B. Smith; J. Am. Chem. Soc. 114, 2567 (1992)
- Biosynthesis of the antibiotic echinosporin by a novel branch of the shikimate pathway: A. Dubeler; Eur. J. Org. Chem. 2002, 983 (2002)
- Echinosporins as new cell cycle inhibitors and apoptosis inducers from marine-derived Streptomyces albogriseolus: C.B. Cui, et al.; Fitoterapia 78, 238 (2007)
- A new stereocontrolled synthetic route to (-)-echinosporin from D-glucose via padwa allenylsulfone [3 + 2]-anionic cycloadditive elimination: J.T. Flasz, et al.; Org. Lett. 14, 3024 (2012)
- Interaction of marine streptomyces compounds with selected cancer drug target proteins by in silico molecular docking studies: A.R. Lankapalli & K. Kannabiran; Interdiscipl. Sci. Comput. Life Sci. 5, 37 (2013)