Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
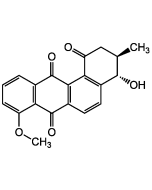
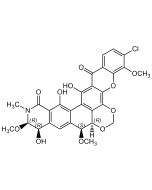
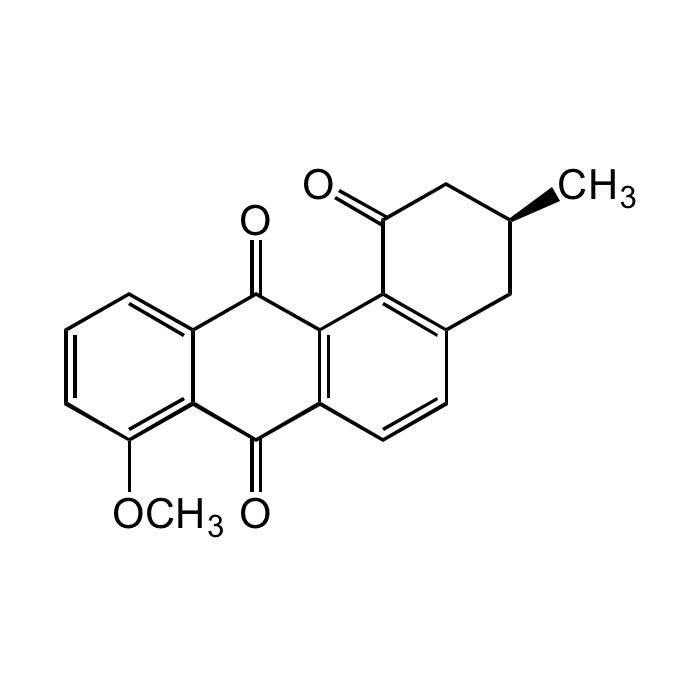
Rubiginone B2
As low as
160
CHF
CHF 160.00
In stock
Only %1 left
BVT-0026-M0011 mgCHF 160.00
| Product Details | |
|---|---|
| Synonyms | (+)-Rubiginone B2; X-14881C; 8-O-Methyl-ochramycinone |
| Product Type | Chemical |
| Properties | |
| Formula |
C20H16O4 |
| MW | 320.3 |
| CAS | 130548-10-6 |
| Source/Host Chemicals | Isolated from Streptomyces sp. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Yellow solid. |
| Solubility | Soluble in DMSO, methanol, acetone or dichloromethane. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | ZUCWNLVDTXGGSU-JTQLQIEISA-N |
| Smiles | O=C1C2=C(C(C(C[C@@H](C)C3)=O)=C3C=C2)C(C4=CC=CC(OC)=C41)=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light when in solution. |
| Use/Stability |
Stable for at least 1 year after receipt when stored at -20°C. After reconstitution protect from light at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Angucyclinone antibiotic.
- Antibacterial and antitumor agent.
- Potentiates the cytotoxicity of vincristine.
- Antimalarial agent.
Product References
- Microbial products XI. Five novel metabolites related to benz[a]anthracene from an unidentified actinomycete designated X-14881: H. Maehr, et al.; J. Antibiot. 35, 1627 (1982)
- Chemical and biological properties of rubiginone, a complex of new antibiotics with vincristine-cytotoxicity potentiating activity: M. Oka, et al.; J. Antibiot. 43, 967 (1990)
- Absolute configuration of the rubiginones and photo-induced oxidation of the C1 hydroxyl of the antibiotics to a ketone: M. Oka, et al.; Tetrahed. Lett. 31, 7473 (1990)
- Angucycline group antibiotics: J. Rohr & R. Thiericke; Nat. Prod. Rep. 9, 103, (1992)
- New biologically active rubiginones from Streptomyces sp.: C. Puder, et al.; J. Antibiot. 53, 329 (2000)
- Angucyclinone Antibiotics: Total Syntheses of YM-181741, (+)-Ochromycinone, (+)-Rubiginone B2, Tetrangomycin and MM-47755: K.P. Kaliappan & V. Ravikumar; J. Org. Chem. 72, 6116 (2007)
- Saccharosporones A, B and C, cytotoxic antimalarial angucyclinones from Saccharopolyspora sp. BCC 21906: C. Boonlarppradab, et al.; J. Antibiot. 66, 305 (2013)