Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
BioViotica
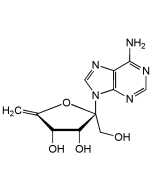
Oxyplicacetin
As low as
130
CHF
CHF 130.00
In stock
Only %1 left
BVT-0031-M0011 mgCHF 130.00

| Product Details | |
|---|---|
| Synonyms | 3′-Hydroxyplicacetin; Cytosaminomycin E |
| Product Type | Chemical |
| Properties | |
| Formula |
C25H35N5O8 |
| MW | 533.6 |
| CAS | 100108-92-7 |
| Source/Host Chemicals | Isolated from Streptomyces sp. |
| Purity Chemicals | ≥98% (NMR) |
| Appearance | White solid. |
| Solubility | Soluble in DMSO or water. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by BioViotica. |
| InChi Key | UYEYXSOGZWDEFY-LJEPMGMESA-N |
| Smiles | CC1O[C@H](C[C@@H](O)[C@@H]1O[C@H]1OC(C)[C@H]([C@H](O)C1O)N(C)C)N1C=CC(NC(=O)C2=CC=C(N)C=C2)=NC1=O |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light when in solution. |
| Use/Stability | Stable for at least 1 year after receipt when stored at +4°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Nucleoside antibiotic (amicetin group).
- Anticoccidial agent.
- Shows broad antibacterial activity.
Product References
- Studies on metabolites produced by Streptomyces ramulosus Tue-34. II. The structural elucidation of oxyplicacetin, a new amicetin: Y. Chen, et al.; Kangshengsu 10, 285 (1985) (Chinese)
- Cytosaminomycins, new anticoccidial agents produced by Streptomyces sp. KO-8119. I. Taxonomy, production, isolation and physico-chemical and biological properties: K. Haneda, et al.; J. Antibiot. 47, 774 (1994)
- Cytosaminomycins, new anticoccidial agents produced by Streptomyces sp. KO-8119. II. Structure elucidation of cytosaminomycins A, B, C and D: K. Shiomi, et al.; J. Antibiot. 47, 782 (1994)
- Characterization of the amicetin biosynthesis gene cluster from Streptomyces vinaceusdrappus NRRL 2363 implicates two alternative strategies for amide bond formation: G. Zhang, et al.; Appl. Environ. Microbiol. 78, 2393 (2012)