Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
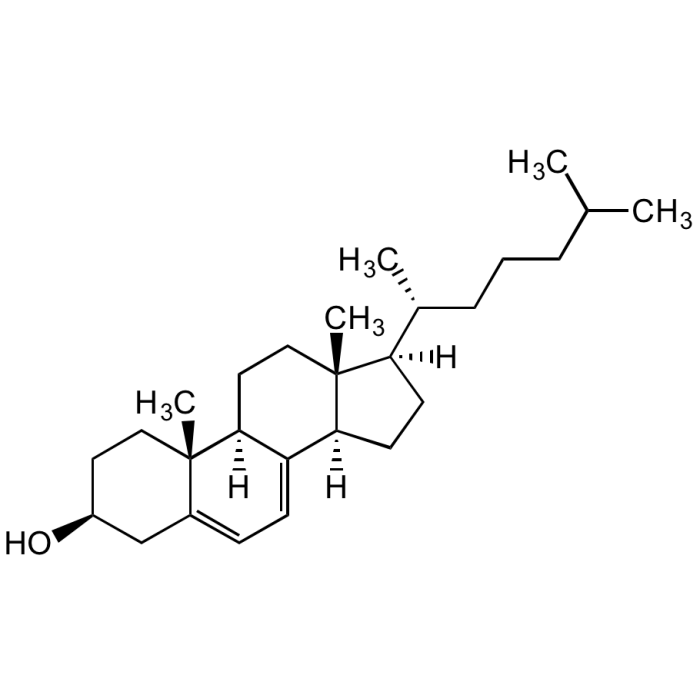
7-Dehydrocholesterol
| Product Details | |
|---|---|
| Synonyms | 7-DHC; NSC 18159; Provitamin D3; Δ7-Cholesterol; (−)-7-Dehydrocholesterol; 3β-Hydroxy-5,7-cholestadiene; 5,7-Cholestadien-3β-ol |
| Product Type | Chemical |
| Properties | |
| Formula | C27H44O |
| MW | 384.64 |
| CAS | 434-16-2 |
| Source/Host Chemicals | Synthetic |
| Purity Chemicals | ≥95% (HPLC) |
| Appearance | White powder. |
| Solubility | Soluble in ethanol, chloroform or ethyl acetate. Slightly soluble in DMSO. Insoluble in water. |
| Identity | Determined by NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | UCTLRSWJYQTBFZ-DDPQNLDTSA-N |
| Smiles | [H][C@@]1(CC[C@@]2([H])C3=CC=C4C[C@@H](O)CC[C@]4(C)[C@@]3([H])CC[C@]12C)[C@H](C)CCCC(C)C |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Precursor of cholesterol and vitamin D3. It is reduced to cholesterol by the enzyme 3β-hydroxysterol-Δ7-reductase (DHCR7) in the last step of cholesterol biosynthesis. Mutations in the gene encoding DHCR7 lead to increased levels of 7-DHC and the neurodevelopmental syndrome Smith-Lemli-Opitz syndrome (SLOS). 7-DHC is used as a biomarker for SLOS screenings. Inhibits human fibroblast sphingomyelinase. Shown to have cytotoxic effects on melanoma cells. In skin, photolysis of 7-DHC by ultraviolet light produces vitamin D3. UV/Vis: λmax 271, 282, 293nm. Recently, 7-Dehydrocholesterol has been shown to inhibit ferroptosis by blocking oxidation of phospholipids, which is the cause of ferroptosis.
(1) T. Okano, et al.; J. Nutr. Sci. Vitaminol. 24, 47 (1978) | (2) J.c. Maziere, et al.; BBRC 100, 1299 (1981) | (3) Q. Xiong, et al.; Chem. Phys. Lipids 115, 1 (2002) | (4) A. Valencia, et al.; Free. Radic. Biol. Med. 41, 1704 (2006) | (5) M. Gelzo, et al.; Free. Radic. Biol. Med. 70, 129 (2014) | (6) W. Liu, et al.; J. Lipid. Res. 55, 329 (2014) | (7) F. Porto Freitas, et al.; Nature (epub ahead of print) (2024)