Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
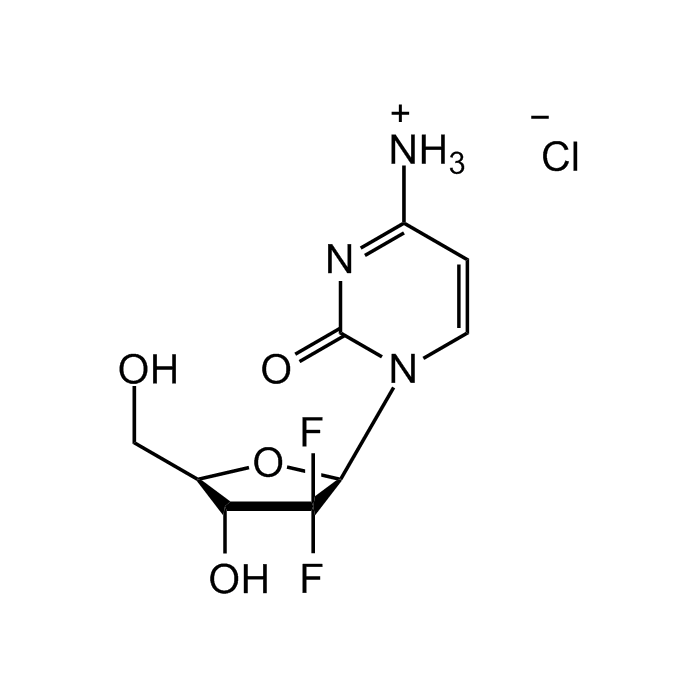
Gemcitabine hydrochloride
| Product Details | |
|---|---|
| Synonyms | 2'-Deoxy-2',2'-difluorocytidine; dFdC; Gemzar (Lilly); LY-188011; dFdC; dFdCyd; NSC613327 |
| Product Type | Chemical |
| Properties | |
| Formula |
C9H11F2N3O4 . HCl |
| MW | 263.20 . 36.46 |
| CAS | 122111-03-9 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in water (20mg/ml) or DMSO (slightly). Insoluble in ethanol. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | OKKDEIYWILRZIA-OSZBKLCCSA-N |
| Smiles | OC[C@@H]1[C@@H](O)C(F)(F)[C@H](N2C=CC([NH3+])=NC2=O)O1.[Cl-] |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +20°C |
| Long Term Storage | +20°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at RT. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Widely used antitumor agent in vitro and in vivo. Deoxycytidine analog with cytotoxic effects. Inhibits DNA synthesis and induces apoptosis through inhibition of ribonucleotide reductase (R1 and R2). Following the uptake of this prodrug into cells by nucleoside transporters it is phosphorylated to its mono(dFdCMP), di(dFdCDP), and triphosphorylated(dFdCTP) forms by deoxycytidine kinase. dFdCDP and dFdCTP are reported to inhibit the activity of ribonucleotide reductase and impede DNA synthesis and repair mechanisms and induce cell death. The triphosphate form is incorporated into DNA which induces masked chain termination and cell death. By specifically inhibiting growth arrest and DNA damage inducible protein 45 a (Gadd45a), a key mediator of active DNA demethylation, inhibits repair-mediated DNA demethylation in a methylation-sensitive reporter assay at concentrations ranging from 34-134nM. This anticancer nucleoside analog inhibits the growth of HL-60 promyelocytic leukemia cells with an LC50 value of 40nM. It inhibits the growth of MX-1 mammary, CX-1, HC-1, GC3, and VRC5 colon, LX-1, Calu-6 and NCI-H460 lung, and HS766T, PaCa-2, PANC-1, and BxPC-3 pancreatic cancer tumors in mouse xenograft models (45-93% inhibition). Also shown to block mitochondrial DNA polymerase γ. Works synergistically with other chemotherapeutic agents to enhance their cytotoxicity. Has also broad antiretroviral activity, decreasing MuLV cell infectivity, a murine AIDS model, in cell culture (EC50 = ~1.5nM) and inhibits the progression of murine AIDS in vivo at a dose of 1-2 mg/kg per day.
(1) L.W. Hertel, et al.; Cancer Res. 50, 4417 (1990) | (2) V. Heinemann, et al.; Cancer Res. 52, 533 (1992) | (3) D.D. Ross & D.P. Cuddy; Biochem. Pharmacol. 48, 1619 (1994) | (5) W. Plunkett, et al.; Semin. Oncol. 22, 3 (1995) | (6) R.L. Merriman, et al.; New Drugs 14, 243 (1996) | (7) J.R. Mackey, et al.; Cancer Res. 58, 4349 (1998) | (8) R.P. McGeary, et al.; Mini Rev. Med. Chem. 8, 1384 (2008) | (9) C.L. Clouser, et al.; PLoS One 6, 1 (2011) | (10) S.W. Hung, et al.; Cancer Lett. 320, 138 (2012)