Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Chemodex
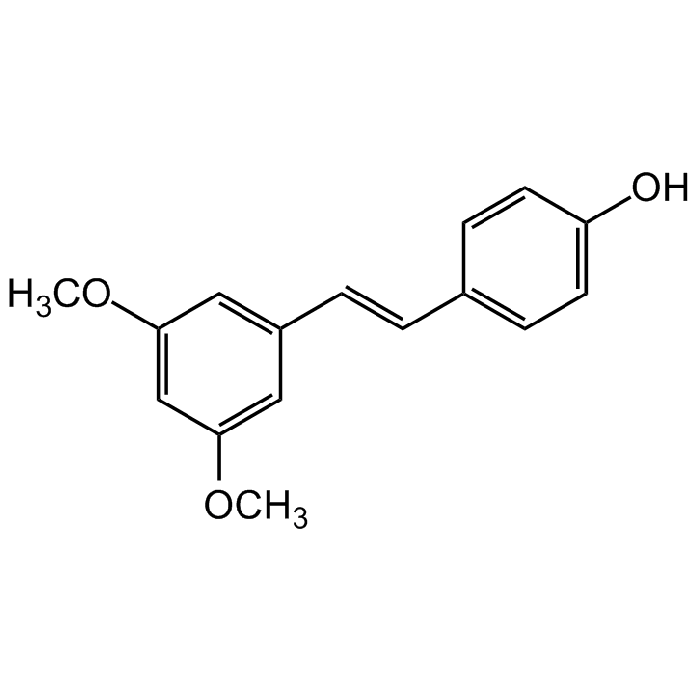
Pterostilbene
| Product Details | |
|---|---|
| Synonyms | trans-3,5-Dimethoxy-4'-Hydroxystilbene; 4-[(1E)-2-(3,5-Dimethoxyphenyl)ethenyl]phenol; 3',5'-Dimethoxy-4-Stilbenol |
| Product Type | Chemical |
| Properties | |
| Formula |
C16H16O3 |
| MW | 256.30 |
| CAS | 537-42-8 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | ≥97% (HPLC) |
| Appearance | Off-white to yellow powder. |
| Solubility | Soluble in DMSO (20 mg/ml), methanol (10 mg/ml), DMF (30 mg/ml) or ethanol (30 mg/ml). Insoluble in water. |
| Identity | Determined by 1H-NMR. |
| Declaration | Manufactured by Chemodex. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet |
| InChi Key | VLEUZFDZJKSGMX-ONEGZZNKSA-N |
| Smiles | OC(C=C1)=CC=C1/C=C/C2=CC(OC)=CC(OC)=C2 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice | Protect from light and moisture. |
| Use/Stability | Stable for at least 2 years after receipt when stored at +4°C. |
| Documents | |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Cell permeable natural methoxylated analog of resveratrol. Antioxidant, antiproliferative, anti-inflammatory, anti-hyperglycemic and anti-diabetic agent. Induces apoptosis. Induces Autophagy. Pterostilbene is a naturally-occurring dimethyl ether analog of resveratrol that is abundant in blueberries. Like resveratrol, pterostilbene acts as a powerful antioxidant, suppresses the synthesis of prostaglandin E2 from lipopolysaccharide-stimulated human peripheral blood mononuclear cells (IC50 = 1.0 μM for pterostilbene, 3.2 μM for resveratrol), and inhibits cell proliferation (IC50 = ~60 μM for both compounds). Very potent inhibitor of cytochrome P450 1A1 (CYP1A1) with Ki=0.57µM. Pterostilbene blocks the activation of ERK1/2, p38 MAPK, and PI3K/Akt signaling pathways, reducing NF-κB and AP-1 transcriptional activation. Moderately inhibits COX-1 and COX-2 (IC50=19.8µM and 83.9µM).
(1) M. Manickam, et al.; J. Nat. Prod. 60, 609 (1997) | (2) A.M. Rimando, et al.; J. Agric. Food Chem. 50, 3453 (2002) | (3) M. Roberti, et al.; J. Med. Chem. 46, 3546 (2003) | (4) R. Amorati, et al.; J. Org. Chem. 69, 7101 (2004) | (5) S. Hougee, et al.; Planta Med. 71, 387 (2005) | (6) P. Ferrer, et al.; Neoplasia 7, 37 (2005) | (7) A.M. Rimando, et al.; J. Agric. Food Chem. 53, 3403 (2005) | (8) R. Mikstacka, et al.; Xenobiotica 36, 269 (2006) | (9) K.A. Roupe, et al.; Curr. Clin. Pharmacol. 1, 81 (2006) | (10) R. Mikstacka, et al.; Mol. Nutr. Food Res. 51, 517 (2007) | (11) M.H. Pan, et al.; J. Agric. Food Chem. 55, 7777 (2007) | (12) B. Billack, et al.; Cell. Mol. Biol. Lett. 13, 553 (2008) | (13) C.M. Remsberg, et al.; Phytother. Res. 22, 169 (2008) | (14) M. Cichocki, et al.; Mol. Nutr. Food Res. 52, S62 (2008) | (15) M.H. Pan, et al.; Carcinogenesis 30, 1234 (2009)