Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
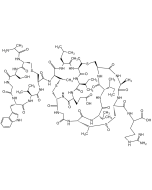
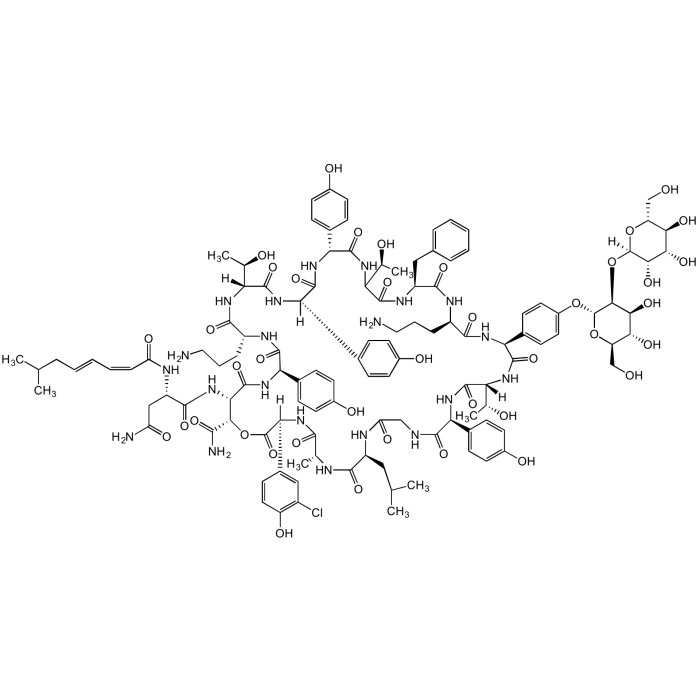
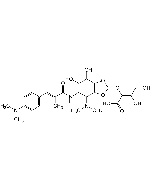
Ramoplanin A2
| Product Details | |
|---|---|
| Synonyms | Antibiotic A16686A2; MDL-62198; Antibiotic A 16686 |
| Product Type | Chemical |
| Properties | |
| Formula |
C119H154ClN21O40 |
| MW | 2554.1 |
| CAS | 81988-88-7 [A2] |
| Source/Host Chemicals | Isolated from Actinoplanes sp. |
| Purity Chemicals | ≥90% (HPLC) |
| Appearance | White to off-white powder. |
| Solubility | Soluble in DMSO or water. |
| Identity | Determined by 1H-NMR and MS. |
| Other Product Data |
Purity Note: The purity is referred exclusively to the main congener(s), the sample also contains minor related congeners. See: Ramoplanin (A-16686), a new glycolipodepsipeptide antibiotic. III. Structure elucidation: R. Ciabatti, et al.; J. Antibiot. 42, 254 (1989) |
| InChi Key | KGZHFKDNSAEOJX-BALZYLSASA-N |
| Smiles | OC[C@H]([C@@H](O)[C@H](O)[C@@H]1O)O[C@]1([H])O[C@@H]2[C@H](O[C@H](CO)[C@@H](O)[C@@H]2O)OC3=CC=C([C@@H]4NC([C@H](NC([C@@H](NC(C(NC([C@@H](C5=CC=C(O)C=C5)NC([C@@](C6=CC=C(O)C=C6)([H])NC([C@@](NC([C@H](NC([C@@H](C7=CC=C(O)C=C7)NC([C@@H](NC([C@H](CC(N)=O)NC(/C=C\C=C\CC(C)C)=O)=O)[C@@H](C(N)=O)OC([C@@](C8=CC(Cl)=C(O)C=C8)([H])NC([C@@H](C)NC([C@H](CC(C)C)NC(CNC([C@H](C9=CC=C(O)C=C9)NC([C@]([C@H](O)C)([H])NC4=O)=O)=O)=O)=O)=O)=O)=O)=O)CCCN)=O)([H])[C@H](O)C)=O)=O)=O)[C@@H](O)C)=O)CC%10=CC=CC=C%10)=O)CCCN)=O)C=C3 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light. Protect from light when in solution. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
-
Glycolipodepsipeptide antibiotic.
-
Complex of structurally related molecules A1, A2 and A3, with ramoplanin A2 as the primary component.
-
Antibacterial and antiviral agent with activity against aerobic and anaerobic Gram-positive bacteria such as Clostridium difficile and antibiotic-resistant enterococci.
-
Inhibits cell wall synthesis and consequently bacterial growth by forming a complex with lipid intermediate II (Lipid II), a key intermediate in peptidoglycan biosynthesis.
-
A-16686, a new antibiotic from Actinoplanes. I. Fermentation, isolation and preliminary physico-chemical characteristics: B. Cavalleri, et al.; J. Antibiot. 37, 309 (1984)
-
A-16686, a new antibiotic from Actinoplanes. II. Biological properties: R. Pallanza, et al.; J. Antibiot. 37, 318 (1984)
-
In vitro evaluation of ramoplanin (MDL 62198, A 16686): S. Dixson, et al.; Eur. J. Clin. Microbiol. Infect. Dis. 7, 819 (1988)
-
In vitro bactericidal activity of the glycopeptide compounds vancomycin, teicoplanin and ramoplanin (A-16686/MDL 62,198): M.D. O'Hare, et al.; J. Chemother. 1, 210 (1989)
-
In-vitro studies with ramoplanin (MDL 62,198): a novel lipoglycopeptide antimicrobial: M.D. O'Hare, et al.; J. Antimicrob. Chemother. 25, 217 (1990)
-
Inhibition of peptidoglycan biosynthesis by ramoplanin: E.A. Somner & P.E. Reynolds; Antimicrob. Agents Chemother. 34, 413 (1990)
-
In-vitro activity of vancomycin, teicoplanin, daptomycin, ramoplanin, MDL 62873 and other agents against staphylococci, enterococci and Clostridium difficile: A. Bartoloni, et al.; J. Antimicrob. Chemother. 26, 627 (1990)
-
Bactericidal activity of ramoplanin against antibiotic-resistant enterococci: C.C. Johnson, et al.; Antimicrob. Agents Chemother. 36, 2342 (1992)
-
In vitro activity of ramoplanin against vancomycin-resistant gram-positive organisms: L.A. Collins, et al.; Antimicrob. Agents Chemother. 37, 1364 (1993)
-
A new structure for the substrate-binding antibiotic ramoplanin: M.C. Lo, et al.; JACS 123, 8640 (2001)
-
Ramoplanin. A 16686, A 16686A, MDL 62198:Adis Comments; Drugs R.D. 3, 271 (2002) (Review)
-
Ramoplanin inhibits bacterial transglycosylases by binding as a dimer to lipid II: Y. Hu, et al.; JACS 125, 8736 (2003)
-
Chemistry and biology of ramoplanin: a lipoglycodepsipeptide with potent antibiotic activity: S. Walker, et al.; Chem. Rev. 105, 449 (2005) (Review)
-
Ramoplanin: a lipoglycodepsipeptide antibiotic: D.K. Farver, et al.; Ann. Pharmacother. 39, 863 (2005) (Review)
-
Lipid II as a target for antibiotics: E. Breukink & B. de Kruijff; Nat. Rev. Drug Discov. 5, 321 (2006) (Review)
-
The mechanism of action of ramoplanin and enduracidin: X. Fang, et al.; Mol. Biosyst. 2, 69 (2006)
-
Cyclic lipodepsipeptides: a new class of antibacterial agents in the battle against resistant bacteria: N. Bionda, et al.; Future Med. Chem. 5, 1311 (2013) (Review)
-
Identification of a two-component regulatory system involved in antimicrobial peptide resistance in Streptococcus pneumoniae: A.M. Diagne, et al. PLoS Pathog. 18, e1010458 (2022)






![NAI-97 [Planosporicin]](https://adipogen.com/media/catalog/product/cache/60eb5af712bc93baae8d55513bd31b01/a/g/ag-cn2-0312_nai-97.png)