Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
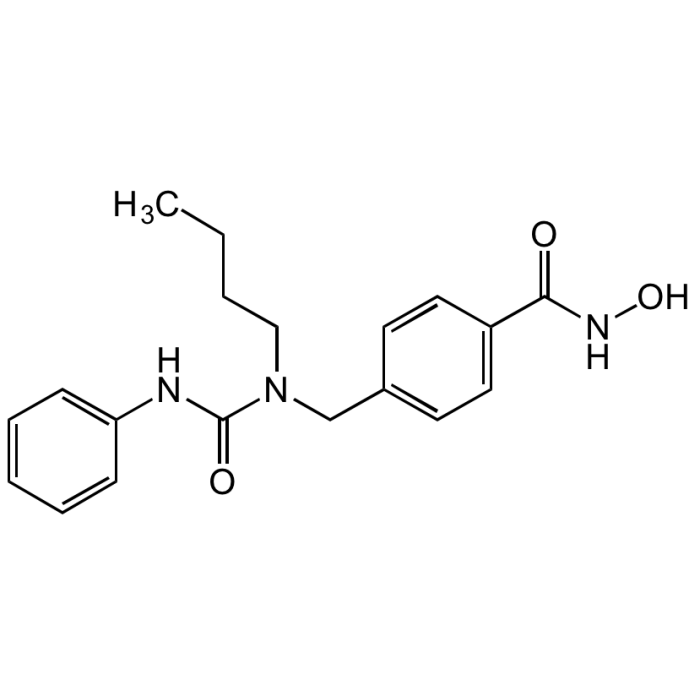
Nexturastat A
As low as
100
CHF
CHF 100.00
In stock
Only %1 left
AG-CR1-3901-M0011 mgCHF 100.00
AG-CR1-3901-M0055 mgCHF 300.00
| Product Details | |
|---|---|
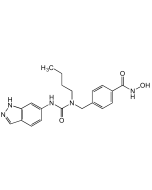
| Synonyms | 4-((1-Butyl-3-phenylureido)methyl)-N-hydroxybenzamide |
| Product Type | Chemical |
| Properties | |
| Formula |
C19H23N3O3 |
| MW | 341.4 |
| CAS | 1403783-31-2 |
| Purity Chemicals | ≥98% (HPLC) |
| Appearance | Light pink solid. |
| Solubility | Soluble in DMSO. Slightly soluble in methanol or ethanol (1mg/ml). Almost insoluble in water. |
| Identity | Determined by 1H-NMR. |
| InChi Key | JZWXMCPARMXZQV-UHFFFAOYSA-N |
| Smiles | CCCCN(CC1=CC=C(C=C1)C(=O)NO)C(=O)NC1=CC=CC=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Cell permeable, potent and selective class IIb HDAC6 inhibitor (IC50 =5.02nM). Displays high selectivity over all other HDACs (IC50=3-10µM).
- Suppresses cell proliferation and promotes apoptosis in B16 cells (GI50=14.3µM) and human lymphoma cells HuT-78. Dose-dependently induces hyperacetylation of α-tubulin in B16 murine melanoma cells without elevating histone H3 acetylation.
- Induces cell cycle arrest in G1.
- HDAC6 deacetylates tubulin, HSP90 and the core histones (H2A, H2B, H3, H4). Histone deacetylases act via the formation of large multiprotein complexes. HDAC6 plays an important role in microtubule-dependent cell motility, transcriptional regulation, degradation of misfolded proteins and cell cycle and is involved in autophagy, inflammation, cancer and neurodegeneration.
Product References
- Selective histone deacetylase 6 inhibitors bearing substituted urea linkers inhibit melanoma cell growth: J.A. Bergman, et al.; J. Med. Chem. 55, 9891 (2012)
- Development and therapeutic implications of selective histone deacetylase 6 inhibitors: J.H. Kalin & J.A Bergman; J. Med. Chem. 56, 6297 (2013)
- Targeting histone deacetylase 6 mediates a dual anti-melanoma effect: Enhanced antitumor immunity and impaired cell proliferation: K.V. Woan, et al.; Mol. Oncol. 9, 1447 (2015)
- Trend of histone deacetylase inhibitors in cancer therapy: Isoform selectivity or multitargeted strategy: L. Zhang, et al.; Med. Res. Rev. 35, 63 (2015) (Review)