Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Innaxon
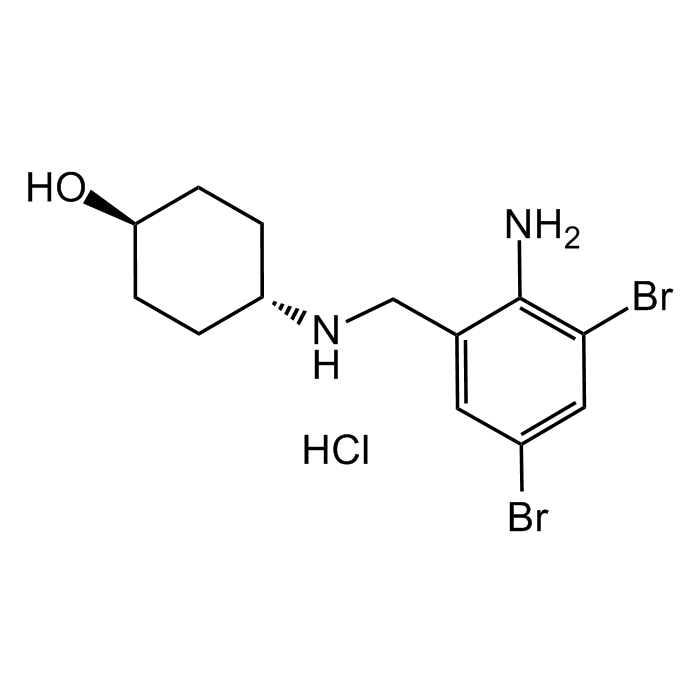
Ambroxol Sterile Solution
| Product Details | |
|---|---|
| Synonyms | Ambroxol Lipodisq™ Sterile Solution; Trans-4-[[(2-amino-3,5-dibromophenyl)methyl]amino]; NA 872; Cyclohexanol |
| Product Type | Chemical |
| Properties | |
| Formula |
C13H18Br2N2O . HCl |
| MW | 378.1 . 36.5 |
| CAS | 23828-92-4 |
| RTECS | GV8423000 |
| Purity Chemicals | ≥95% (HPLC) |
| Appearance | Colourless clear aqueous solution |
| Solubility | Soluble in water, PBS, Tris and other physiological solutions as formulated in a proprietary, thermostable, aqueous lipid nanoparticulate formulation (Lipodisq). |
| Reconstitution | Avoid the use of buffers with divalent ions such as Ca or Mg or pH <6.5 or >8.0, which can cause particle instability. Unformulated ambroxol is soluble in DMF, DMSO or ethanol . |
| Formulation | Liquid, detergent-free discoidal nano-formulation made of styrene-maleic acid lipid particles (SMALP), lecithin and sterile water. |
| Concentration | 1mg/ml (0.1% w/vol) |
| Biological Activity |
Discoidal nano-particles can incorporate hydrophobic, poorly water-soluble compounds, such as lipids, lipoproteins and glycolipids. - Cell culture tested (human macrophage cell line) (MTT). - Recommended starting dilution: 1:200 or higher. - Optimal working concentrations depend on the applications and need to be determined. - Published procedures using Lipodisq formulations (Curcumin and IAXO TLR4 antagonists) in vivo rodent models at 3-10mg/kg. Recommended route of administration is subcutaneous (s.c.) with oral or nasal application as a possible alternative, which needs to be optimized. |
| Declaration | Manufactured by Innaxon. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet: Our product description may differ slightly from the original manufacturers product datasheet.
|
| InChi Key | QNVKOSLOVOTXKF-PFWPSKEQSA-N |
| Smiles | O[C@H]1CC[C@H](NCC2=CC(Br)=CC(Br)=C2N)CC1.Cl |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice |
Keep sterile. Avoid skin and eye contact. |
| Use/Stability | Stable for at least 1 year after receipt when stored at +4°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
- Ambroxol Sterile Solution is a ready-to-use nano-formulated aqueous solution.
- Ambroxol is a mucolytic agent used in the treatment of respiratory diseases. Ambroxol is a basic (pKa = 9.01) cationic drug with lipophilic properties (logP = 2.9), enabling it to act as a lysosomotropic agent. In addition, ambroxol exhibits a novel mechanism by accumulating in lamellar bodies and acting as a lysosomal secretagogue. A wide range of pharmacological effects of ambroxol has been confirmed, including mucus regulation, anti-inflammatory, reduction of arachidonic acid metabolites and pro-inflammatory cytokines, and antioxidant properties. In addition, ambroxol aids in the enhancement of local defense molecules involved in respiratory viral replication. Ambroxol is a sodium channel blocker and mucolytic agent with antioxidant, anti-viral and anti-inflammatory properties. Inhibits tetrodotoxin (TTX)-resistant channels more potently than TTX-sensitive subtypes. Inhibits the release of histamine, leukotrienes and cytokines from human leukocytes and mast cells. Inhibits viral replication and improves the survival rate of mice infected with influenza (H3N2) virus. It is a candidate for use as an anti-COVID19 therapeutic.
- Ambroxol Lipodisq is based on a nanoparticle (11-40nm) drug delivery system comprising a discoidal phospholipid bilayer membrane stabilized by a chaperone molecule annulus. Internal properties of the phospholipid membrane support the disposition and stabilization of drug molecule candidates and preserve the native conformation of membrane molecules. The resulting encapsulated actives are rendered water-soluble and specialized for intra-cellular penetration/delivery via endosomal uptake mechanisms. Lipodisq solutions show a good safety profile and are suitable for in vitro and in vivo investigations.
- Ambroxol in the 21st century: pharmacological and clinical update: M. Malerba & B. Ragnoli; Expert Opin. Drug. Metab. Toxicol. 4, 1119 (2008) (Review)
- Ambroxol as a novel disease-modifying treatment for Parkinson’s disease dementia: protocol for a single centre, randomized, double-blind, placebo-controlled trial: C.R.A. Silveira, et al.; BMC Neurol. 19, 20 (2019)
- Azithromycin and ambroxol as potential pharmacotherapy for SARS-CoV-2: M. Alkotaji; Int. J.Antimicrob. Agents 56, 106192 (2020)
- Topical Treatments and Their Molecular/Cellular Mechanisms in Patients with Peripheral Neuropathic Pain-Narrative: M. Kocot-Kępska, et al.; Pharmaceutics 13, 450 (2021) (Review)
- Inhibition of acid sphingomyelinase by ambroxol prevents SARS-CoV-2 entry into epithelial cells: A. Carpinteiro, et al.; J. Biol. Chem. 296, 100701 (2021)
General References for Lipodisq™ Technology:
- Responsive Hydrophobically Associating Polymers: A Review of Structure and Properties: S.R. Tonge & B.J. Tighe; Adv. Drug Deliv. Rev. 53, 109 (2001)
- Detergent-free formation and physico-chemical characterization of nanosized lipidpolymer complexes: Lipodisq; M.C. Orwick, et al.; Angew. Chem. 51, 4653 (2012)
- Physicochemical Characterization, Toxicity and In Vivo Biodistribution Studies of a Discoidal, Lipid-Based Drug Delivery Vehicle: Lipodisq Nanoparticles Containing Doxorubicin: M.L. Torgersen, et al.; J. Biomed. Nanotechnol. 16, 41 (2020)
- Applications of Synthetic Polymer Discoidal Lipid Nanoparticles to Biomedical Research: M. Tanaka; Chem. Pharm. Bull. 70, 507 (2022)
- Mechanisms of Formation, Structure, and Dynamics of Lipoprotein Discs Stabilized by Amphiphilic Copolymers: A Comprehensive Review: P.S. Orekhov, et al.; Nanomaterials 12, 361 (2022)