Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Innaxon
Umifenovir Sterile Solution
| Product Details | |
|---|---|
| Synonyms | Umifenovir Lipodisq™ Sterile Solution; Arbidol; ARB |
| Product Type | Chemical |
| Properties | |
| Formula |
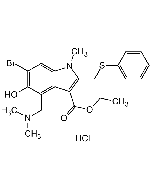
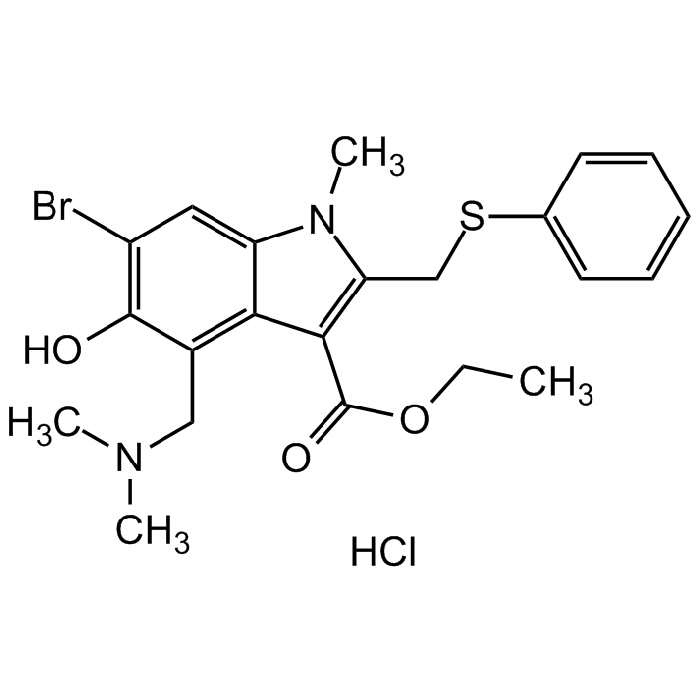
C22H25BrN2O3S . HCl |
| MW | 477.4 . 36.5 |
| CAS | 131707-23-8 |
| RTECS | NL5998075 |
| Purity Chemicals | ≥95% (HPLC) |
| Appearance | Light yellow coloured clear aqueous solution |
| Solubility | Soluble in water, PBS, Tris and other physiological solutions as formulated in a proprietary, thermostable, aqueous lipid nanoparticulate formulation (Lipodisq). |
| Reconstitution | Avoid the use of buffers with divalent ions such as Ca or Mg or pH <6.5 or >8.0, which can cause particle instability. Unformulated umifenovir is soluble in DMF, DMSO or ethanol. |
| Formulation | Liquid, detergent-free discoidal nano-formulation made of styrene-maleic acid lipid particles (SMALP), lecithin and sterile water. |
| Concentration | 1mg/ml (0.1% w/vol) |
| Biological Activity |
Discoidal nano-particles can incorporate hydrophobic, poorly water-soluble compounds, such as lipids, lipoproteins and glycolipids. - Cell culture tested (human macrophage cell line) (MTT). - Recommended starting dilution: 1:200 or higher. - Optimal working concentrations depend on the applications and need to be determined. - Published procedures using Lipodisq formulations (Curcumin and IAXO TLR4 antagonists) in vivo rodent models at 3-10mg/kg. Recommended route of administration is subcutaneous (s.c.) with oral or nasal application as a possible alternative, which needs to be optimized. |
| Declaration | Manufactured by Innaxon. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet: Our product description may differ slightly from the original manufacturers product datasheet.
|
| InChi Key | OMZHXQXQJGCSKN-UHFFFAOYSA-N |
| Smiles | BrC1=C(O)C(CN(C)C)=C(C(C(OCC)=O)=C(CSC2=CC=CC=C2)N3C)C3=C1.Cl |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep sterile. Avoid skin and eye contact. |
| Use/Stability | Stable for at least 1 year after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
- Umifenovir Sterile Solution is a ready-to-use nano-formulated aqueous solution.
- Umifenovir (Arbidol) is known to have broad-spectrum anti-viral activity and has earlier been approved in China and Russia for treating influenza, SARS, and Lassa viruses. It has been tested in multiple clinical studies as a candidate for use as an anti-COVID19 therapeutic and has been suggested to act at the entry stage and at the post-entry stages by preventing viral attachment and inhibiting the release of virus particles from intracellular vesicles, respectively. In a recent phase III, clinical study Umifenovir met the primary and secondary endpoint criteria. It has been shown to efficacious, safe and well-tolerated at the tested dosage.
- Umifenovir Lipodisq is based on a nanoparticle (11-40nm) drug delivery system comprising a discoidal phospholipid bilayer membrane stabilized by a chaperone molecule annulus. Internal properties of the phospholipid membrane support the disposition and stabilization of drug molecule candidates and preserve the native conformation of membrane molecules. The resulting encapsulated actives are rendered water-soluble and specialized for intra-cellular penetration/delivery via endosomal uptake mechanisms. Lipodisq solutions show a good safety profile and are suitable for in vitro and in vivo investigations.
- Arbidol: A broad-spectrum antiviral compound that blocks viral fusion: Y.S. Boriskin, et al.; Curr. Med. Chem. 15, 997 (2008) (Review)
- Arbidol as a broad-spectrum antiviral: an update: J. Blaising, et al.; Antiviral Res. 107, 84 (2014) (Review)
- Potential treatment methods targeting 2019-nCoV infection: L.U. Zheng, et al.; Eur. J. Med. Chem. 205, 112687 (2020)
- Phase III, Randomized, Double-blind, Placebo controlled trial of Efficacy, Safety and Tolerability of Antiviral drug Umifenovir vs Standard care of therapy in non-severe COVID-19 patients: R. Ramachandran, et al.; Int. J. Infect. Dis. 115, 62 (2022)
General References for Lipodisq™ Technology:
- Responsive Hydrophobically Associating Polymers: A Review of Structure and Properties: S.R. Tonge & B.J. Tighe; Adv. Drug Deliv. Rev. 53, 109 (2001)
- Detergent-free formation and physico-chemical characterization of nanosized lipidpolymer complexes: Lipodisq; M.C. Orwick, et al.; Angew. Chem. 51, 4653 (2012)
- Physicochemical Characterization, Toxicity and In Vivo Biodistribution Studies of a Discoidal, Lipid-Based Drug Delivery Vehicle: Lipodisq Nanoparticles Containing Doxorubicin: M.L. Torgersen, et al.; J. Biomed. Nanotechnol. 16, 41 (2020)
- Applications of Synthetic Polymer Discoidal Lipid Nanoparticles to Biomedical Research: M. Tanaka; Chem. Pharm. Bull. 70, 507 (2022)
- Mechanisms of Formation, Structure, and Dynamics of Lipoprotein Discs Stabilized by Amphiphilic Copolymers: A Comprehensive Review: P.S. Orekhov, et al.; Nanomaterials 12, 361 (2022)