Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Innaxon
Oxyresveratrol Sterile Solution
| Product Details | |
|---|---|
| Synonyms | Oxyresveratrol Lipodisq™ Sterile Solution; trans-2',3,4',5-Tetramethoxystilbene |
| Product Type | Chemical |
| Properties | |
| Formula |
C14H12O4 |
| MW | 244.2 |
| CAS | 29700-22-9 |
| Source/Host Chemicals | Synthetic. |
| Purity Chemicals | >95% (HPLC) |
| Appearance | Light amber coloured clear aqueous solution |
| Solubility | Soluble in water, PBS, Tris and other physiological solutions as formulated in a proprietary, thermostable, aqueous lipid nanoparticulate formulation (Lipodisq). |
| Reconstitution | Avoid the use of buffers with divalent ions such as Ca or Mg or pH <6.5 or >8.0, which can cause particle instability. Unformulated oxyresveratrol is soluble in chloroform. |
| Formulation | Liquid, detergent-free discoidal nano-formulation made of styrene-maleic acid lipid particles (SMALP), lecithin and sterile water. |
| Concentration | 1mg/ml (0.1% w/vol) |
| Biological Activity |
Discoidal nano-particles can incorporate hydrophobic, poorly water-soluble compounds, such as lipids, lipoproteins and glycolipids. - Cell culture tested (human macrophage cell line) (MTT). - Recommended starting dilution: 1:200 or higher. - Optimal working concentrations depend on the applications and need to be determined. - Published procedures using Lipodisq formulations (Curcumin and IAXO TLR4 antagonists) in vivo rodent models at 3-10mg/kg. Recommended route of administration is subcutaneous (s.c.) with oral or nasal application as a possible alternative, which needs to be optimized. |
| Declaration | Manufactured by Innaxon. |
| Other Product Data |
Click here for Original Manufacturer Product Datasheet: Our product description may differ slightly from the original manufacturers product datasheet.
|
| InChi Key | PDHAOJSHSJQANO-UPHRSURJSA-N |
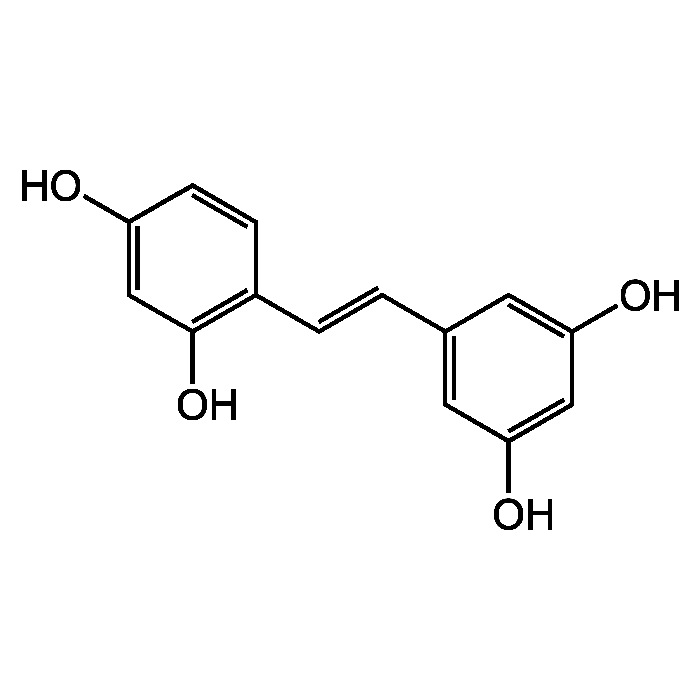
| Smiles | OC1=CC(O)=C(\C=C\C2=CC(O)=CC(O)=C2)C=C1 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | +4°C |
| Handling Advice |
Keep sterile. Avoid skin and eye contact. |
| Use/Stability | Stable for at least 1 year after receipt when stored at +4°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
- Oxyresveratrol Sterile Solution is a ready-to-use nano-formulated aqueous solution.
- Oxyresveratrol is a naturally occurring analog of resveratrol. Potent antioxidant and free-radical scavenger. Shown to have neuroprotective activity against cerebral ischemia and against traumatic injury. Apoptosis inhibitor in transient cerebral ischemia. Inhibits viral DNA replication and late viral protein synthesis. Has depigmenting effects by effectively inhibiting tyrosinase activity, which catalyzes the rate-limiting step in synthesizing melanin pigments. Has anti-inflammatory properties based on inhibition of iNOS expression through down-regulation of NF-kB binding activity and significant inhibition of COX-2 activity. Anti-hyperlipidemic agent.
- Oxyresveratrol Lipodisq is based on a nanoparticle (11-40nm) drug delivery system comprising a discoidal phospholipid bilayer membrane stabilized by a chaperone molecule annulus. Internal properties of the phospholipid membrane support the disposition and stabilization of drug molecule candidates and preserve the native conformation of membrane molecules. The resulting encapsulated actives are rendered water-soluble and specialized for intra-cellular penetration/delivery via endosomal uptake mechanisms. Lipodisq solutions show a good safety profile and are suitable for in vitro and in vivo investigations.
- Oxyresveratrol Supplementation to C57bl/6 Mice Fed with a High-Fat Diet Ameliorates Obesity-Associated Symptoms: H.Y. Tan, et al.; 9, 147 Nutrients (2017)
- Oxyresveratrol Inhibits R848-Induced Pro-Inflammatory Mediators Release by Human Dendritic Cells Even When Embedded in PLGA Nanoparticles: S.C. Gaglio, et al.; Molecules 26, 2106 (2021)
- Oxyresveratrol Ameliorates Dextran Sulfate Sodium-Induced Colitis in Rats by Suppressing Inflammation: J. Yeom, et al.; Molecules 26, 2630 (2021)
- Oxyresveratrol: Sources, Productions, Biological Activities, Pharmacokinetics, and Delivery Systems: K. Likhitwitayawuid; Molecules 26, 4212 (2021)
- Oxyresveratrol-Loaded PLGA Nanoparticles Inhibit Oxygen Free Radical Production by Human Monocytes: Role in Nanoparticle Biocompatibility: M. Donini, et al.; Molecules 26, 4351 (2021)
General References for Lipodisq™ Technology:
- Responsive Hydrophobically Associating Polymers: A Review of Structure and Properties: S.R. Tonge & B.J. Tighe; Adv. Drug Deliv. Rev. 53, 109 (2001)
- Detergent-free formation and physico-chemical characterization of nanosized lipidpolymer complexes: Lipodisq; M.C. Orwick, et al.; Angew. Chem. 51, 4653 (2012)
- Physicochemical Characterization, Toxicity and In Vivo Biodistribution Studies of a Discoidal, Lipid-Based Drug Delivery Vehicle: Lipodisq Nanoparticles Containing Doxorubicin: M.L. Torgersen, et al.; J. Biomed. Nanotechnol. 16, 41 (2020)
- Applications of Synthetic Polymer Discoidal Lipid Nanoparticles to Biomedical Research: M. Tanaka; Chem. Pharm. Bull. 70, 507 (2022)
- Mechanisms of Formation, Structure, and Dynamics of Lipoprotein Discs Stabilized by Amphiphilic Copolymers: A Comprehensive Review: P.S. Orekhov, et al.; Nanomaterials 12, 361 (2022)