Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
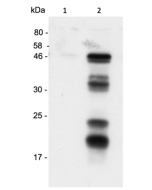
anti-Gasdermin D (mouse), pAb (IN110)

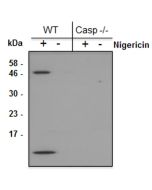
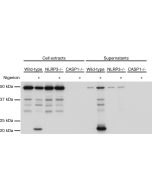
Method: Gasdermin D is analyzed by Western blot in cell extracts of bone marrow-derived macrophage cells (BMDMs) (WT, Gasdermin -/- or Asc -/-) treated with LPS (50ng/ml) for 3h and +/- Nigericin (5μM for 2.5h, Prod. No. AG-CN2-0020). Cell extracts are separated by SDS-PAGE under reducing conditions, transferred to nitrocellulose and incubated with anti-Gasdermin D (mouse), pAb (IN110) (0.5µg/ml). After addition of an anti-Guinea Pig secondary antibody coupled to HRP (1/5000), proteins are visualized by a chemiluminescence detection system.
Picture courtesy of Prof. Olaf Gross, University Medical Center Freiburg, Germany
| Product Details | |
|---|---|
| Synonyms | Gasdermin Domain-containing Protein 1; Gasdermin-D (C-terminal); GSDMD-CT; Gsdmd |
| Product Type | Polyclonal Antibody |
| Properties | |
| Source/Host | Guinea pig |
| Immunogen/Antigen | Recombinant mouse gasdermin D (C-terminus). |
| Application |
Western Blot: (1:2'000)* *Note: We recommend a starting dilution of 1:2'000 and to optimize the dilution depending on your cells/tissues. |
| Crossreactivity | Mouse |
| Specificity |
Recognizes full-length and cleaved C-terminus domain of mouse gasdermin D. Does not cross-react with human gasdermin D. |
| Purity | ≥95% (SDS-PAGE) |
| Purity Detail | Protein A affinity purified. |
| Concentration | 1mg/ml |
| Formulation | Liquid. In PBS containing 10% glycerol and 0.02% sodium azide. |
| Other Product Data |
UniProt link Q9D8T2: Gasdermin D (mouse) |
| Accession Number | Q9D8T2 |
| Shipping and Handling | |
| Shipping | BLUE ICE |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
After opening, prepare aliquots and store at -20°C. Avoid freeze/thaw cycles. |
| Use/Stability | Stable for at least 1 year after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
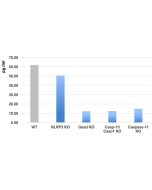
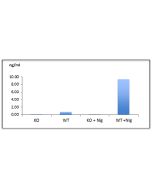
Inflammasomes are multimeric protein complexes that comprise a sensor (e.g. NLRP3), an adaptor (ASC/Pycard) and the procaspase-1. An inflammasome assembles in response to a diverse range of pathogen-associated or danger-associated molecular patterns (PAMPs or DAMPs). The inflammasome platform leads to activation of caspase-1, which further induces maturation of interleukin-1β and -18 (IL-1β and IL-18) through proteolytic cleavage of pro-IL-1β and pro-IL-18. Activated caspase-1, and also the recently characterized caspase-11 non-canonical inflammasome pathway, also cleave the intracellular gasdermin D, which leads to a particular form of inflammatory cell death called pyroptosis. The gasdermin family members contain N-terminal domains that are capable of forming membrane pores to induce cytolysis, whereas the C-terminal domains of gasdermins function as inhibitors of such cytolysis through intramolecular domain association. Caspase-1 or -11 cleavage of gasdermin D is required for regulation of pyroptosis: upon protease cleavage of the gasdermin N- and C-domain linker, the disruption of the intramolecular domain interaction in the presence of lipids releases the N-domain to assemble oligomeric membrane pores that trigger cell death. Gasdermin D seems to be a key effector in the LPS-induced lethal sepsis.
- RNA viruses promote activation of the NLRP3 inflammasome through cytopathogenic effect-induced potassium efflux: L. Silva da Costa, et al.; Cell Death Dis. 10, 346 (2019)
- IKKβ is required for the formation of the NLRP3 inflammasome: S.K. Nanda, et al.; EMBO Rep. e50743 (2021)
- GSK3β mediates the spatiotemporal dynamics of NLRP3 inflammasome activation: S. Arumugam, et al.; Cell Death Differ. ahead of print (2022)
- dsDNA-induced AIM2 pyroptosis halts aberrant inflammation during rhabdomyolysis-induced acute kidney injury: C. Baatarjav, et al.; Cell Death Differ. 29, 2487 (2022)
- Neuronal NLRP3 is a parkin substrate that drives neurodegeneration in Parkinson's disease: N. Panicker, et al.; Neuron 110, 2422 (2022)