Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
AdipoGen Life Sciences
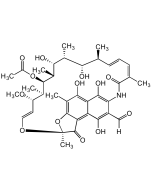
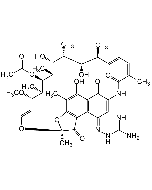
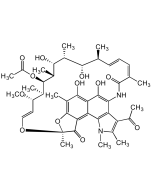
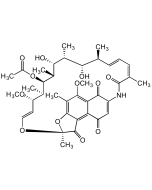
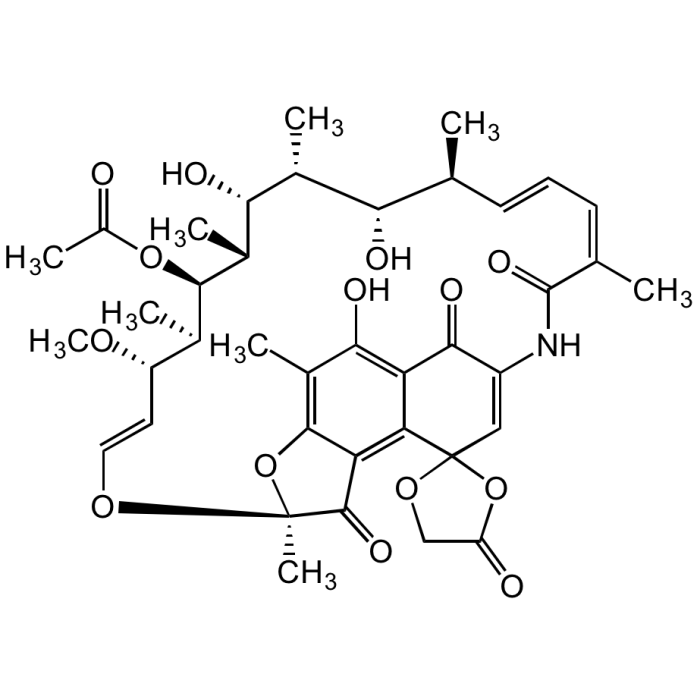
Rifamycin O
As low as
90
CHF
CHF 90.00
In stock
Only %1 left
AG-CN2-0333-M05050 mgCHF 90.00
AG-CN2-0333-M500500 mgCHF 490.00
| Product Details | |
|---|---|
| Synonyms | 4-O-(Carboxymethyl)-1-deoxy-1,4-dihydro-4-hydroxy-1-oxo-rifamycin γ-lactone; NSC 182391 |
| Product Type | Chemical |
| Properties | |
| Formula |
C39H47NO14 |
| MW | 753.8 |
| CAS | 14487-05-9 |
| Source/Host Chemicals | Semisynthetic. |
| Purity Chemicals | ≥90% (HPLC) |
| Appearance | Beige powder. |
| Solubility | Soluble in DMSO, aqueous acetonitrile or ethanol. |
| Identity | Determined by 1H-NMR and MS. |
| InChi Key | RAFHKEAPVIWLJC-MENVPHBUSA-N |
| Smiles | O=C1C(NC(/C(C)=C\C=C\[C@H](C)[C@H](O)[C@@H](C)[C@H]([C@@H](C)[C@@H]([C@H](C)[C@H](/C=C/O2)OC)OC(C)=O)O)=O)=CC3(OCC(O3)=O)C4=C5C(O[C@@]2(C)C5=O)=C(C)C(O)=C41 |
| Shipping and Handling | |
| Shipping | AMBIENT |
| Short Term Storage | +4°C |
| Long Term Storage | -20°C |
| Handling Advice |
Keep cool and dry. Protect from light. Protect from light when in solution. |
| Use/Stability | Stable for at least 2 years after receipt when stored at -20°C. |
| Documents | |
| MSDS |
 Download PDF Download PDF |
| Product Specification Sheet | |
| Datasheet |
 Download PDF Download PDF |
Description
- Ansamycin antibiotic.
- Selective inhibitor of bacterial DNA-dependent RNA polymerase (RNAP).
- Effective against mycobacteria and therefore used in research of tuberculosis, leprosy and Mycobacterium avium complex (MAC) infections.
Product References
- Production of Rifamycin O by Streptomyces 4107 A2: S. Sugawara, et al.; J. Antibiot. 17, 29 (1964)
- Rifamycins: A General View: S. Riva & L.G. Silvestri; Ann. Rev. Microbiol. 26, 199 (1972)
- The constitution and configuration of rifamycins B, O, S and SV: W. Oppolzer & V. Prelog; Helv. Chim. Acta 56, 2287 (1973)
- Comprehensive study on structure-activity relationships of rifamycins: discussion of molecular and crystal structure and spectroscopic and thermochemical properties of rifamycin O: A. Bacchi, et al.; J. Med. Chem. 41, 2319 (1998)
- Rifamycins-Mode of Action, Resistance, and Biosynthesis: H.G. Floss & T.-W. Yu; Chem. Rev. 105, 621 (2005)