Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Lipodisq™ - Novel Lipid/Polymer Nanoparticles
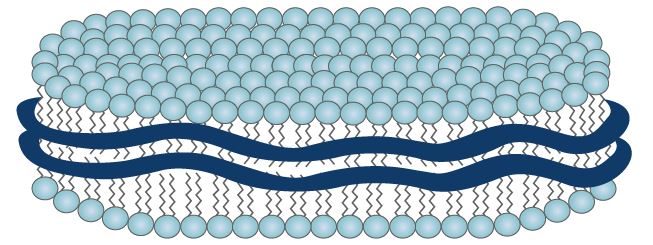
Lipodisq™ are novel lipid/polymer nanoparticles that have been developed as mimics of naturally-occurring high-density lipoproteins (HDL). Lipodisq™ particles are in the size range of 11 to 40 nm in diameter enabling enhanced penetration and diffusion into membranes. These nanodisc lipid particles are composed of a hydrophilic shell and hydrophobic core in which hydrophobic active agents can be carried and protected.
Lipodisq™ polymers are available as 4 defined structures (1:1, 2:1, 3:1, and 4:1 Styrene to MaleicAcid (SMA) rations) each individually operational within a selected pH range for optimal working conditions. Components are batch-tested for Lipodisq™ formation using buffer systems available, which are tested for nano-formulated drug analysis by Dynamic Light Scattering (DLS). Lipodisq™ formation is highly efficient and nanodiscs show a good safety profile and are suitable for in vitro and in vivo (in experimental animals) investigations.
The size and shape of the chaperone molecule is a critical factor in Lipodisq™ formation and also defines the properties of the particle, i.e. particle size and/or its biodegradability. Internal properties of the phospholipid membrane support the disposition and stabilization of drug molecule candidates and preserve the native conformation of membrane-bound molecules. The resulting encapsulated actives are rendered water-soluble and optimized for intracellular penetration/delivery via endosomal uptake mechanisms.
FIGURE: Nanoparticle (11-40 nm) drug delivery system comprising a discoidal phospholipid bilayer membrane stabilized by an annular chaperone molecule.
LITERATURE REFERENCES
- Responsive Hydrophobically Associating Polymers: A Review of Structure and Properties: S.R. Tonge & B.J. Tighe; Adv. Drug Deliv. Rev. 53, 109 (2001)
- Detergent-free formation and physico-chemical characterization of nanosized lipidpolymer complexes: Lipodisq; M.C. Orwick, et al.; Angew. Chem. 51, 4653 (2012)
- The styrene–maleic acid copolymer: a versatile tool in membrane research: J.M. Doerr, et al.; Eur. Biophys. J. 45, 3 (2016)
- Effects of charged lipids on the physicochemical and biological properties of lipid–styrene maleic acid copolymer discoidal particles: M. Tanaka, et al.; Biochim. Biophys. Acta. Biomembr. 1862, 183209 (2020)
- Physico-chemical Characterization, Toxicity and In Vivo Biodistribution Studies of a Discoidal, Lipid-Based Drug Delivery Vehicle: Lipodisq Nanoparticles Containing Doxorubicin: M.L. Torgersen, et al.; J. Biomed. Nanotechnol. 16, 41 (2020)
Not for cosmetic, diagnostic or therapeutic human or veterinary use!
Lipid Nanoparticles
1,2-Dimyristoyl-sn-glycero-3-phosphocholine (DMPC) is a synthetic phospholipid utilized in liposomes and lipid bilayers for the study of biological membranes. Its utility extends to investigating the influence of lipids on enzymatic activity and protein function when formulated into nanodiscs, LNP or liposomes. Phosphatidylcholines are zwitterionic phospholipids containing choline as part of the polar head group. At physiological pH, phosphocholine has an overall neutral charge. Furthermore, this compound has proven valuable in exploring the interplay between lipids and proteins, elucidating their interactions, and unraveling the impact of lipids on protein structure and functionality.
Lipodisq™ are nanosized lipid-based discoidal particles that can be manufactured with P(SMA)/ SMALP and lipids such as DMPC (14:0 PC) (DMPC: IAX-700-400) to incorporate hydrophobic, poorly water-soluble active compounds, such as peptides, lipids, lipoproteins, transmembrane proteins and glycolipids. Applications of Lipodisq™ include functional and structural characterization of the cargo and drug delivery with improved bioavailability, and biological half-life in vivo (PD/PK) or delivery of antigens preserved in their native conformation for immunization purposes.
| Product Name | PID |
| SMA-100 | IAX-700-201 |
| SMA-200 | IAX-700-202 |
| SMA-300 | IAX-700-203 |
| SMA-400 | IAX-700-204 |
| DMPC (1,2-Dimyristoyl-sn-glycero-3-phosphocholine) (14:0 PC) | IAX-700-400 |
Compounds powered by Lipodisq™ Features
- Actives in Lipodisq™ are biosynthetic water-soluble nanodiscs prepared under SOPs using selected optimized lipid compositions for stable, high-loading capacity of encapsulated active ingredients.
- Actives in Lipodisq™ are detergent-free nano-formulations made of styrene-maleic acid copolymer-lipid particles (SMALP).
- Actives in Lipodisq™ retain the biological activity of the active compound with enhanced bioavailability.
- Lipodisq™ solutions show a good safety profile and are suitable for in vitro and in vivo investigations.
SPECIAL Features of Innaxon Compounds powered by Lipodisq™
- >1011 particles per ml as determined by Dynamic Light Scattering (DLS).
- Tested in cell culture (human macrophage cell line as tested by MTT viability test).
- Formulations are soluble in water, PBS, Tris and other physiological solutions as formulated in a proprietary, thermostable, aqueous lipid nanoparticulate formulation.
- Formulations are certified sterile solutions with a physiological pH range.
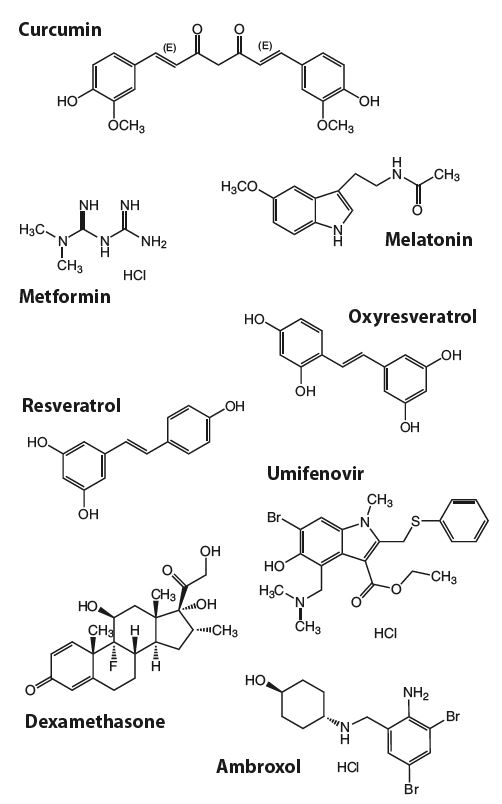
Selection of Antiviral and Immunomodulating Compounds powered by Lipodisq™:
New Ready-to-use Nano-formulated Aqueous Solutions (1 mg/ml)
| Product Name | PID |
| Lipodisq™ Control Sterile Solution | IAX-700-100 |
| Curcumin (high purity) Sterile Solution | IAX-700-101 |
| Melatonin Sterile Solution | IAX-700-102 |
| Mertformin Sterile Solution | IAX-700-103 |
| Oxyresveratrol Sterile Solution | IAX-700-104 |
| Resveratrol Sterile Solution | IAX-700-105 |
| Umifenovir Sterile Solution | IAX-700-106 |
| Dexamethasone Sterile Solution | IAX-700-107 |
| Ambroxol Sterile Solution | IAX-700-108 |
| Retinoic Acid Sterile Solution | IAX-700-109 |
LEGAL CLAIM: The use of styrene maleic acid copolymer-phospholipid nanoparticles (Lipodisq™ Technology) and active agents contained therein is covered by one or more of the following patents owned by Malvern Cosmeceutics Limited: AU2006253886, CA2611144, CN101184473B, EP1890675, GB2426703, IN261468, JP5142898, US8623414 and WO/2021/005340A1 pending.
For All Innaxon Reagents
|
More Information
|
Downloadable Flyer
|
|
|
Lipodisq™ Styrene: Maleic Acid Copolymer [SMA] 2026 - Toolkit for Drug Delivery of API and for Membrane Protein Study Lipodisq™ reagents are novel polymers containing styrene and maleic acid monomer units. Lipodisq™ polymers are capable of forming nano-sized lipid-based discoidal particles (nanodiscs or SMA-Lipid Particles / SMA-LP) suitable for incorporating membrane proteins. The Lipodisq™ technology can solubilize commonly used lipids such as dimyristoylphosphatidylcholine (DMPC) with hydrophobic active ingredients (API) without the use of detergents. |
Download |
|
|
Innaxon Lipodisq Product Flyer 2022 Lipodisq™ are novel lipid/polymer nanoparticles that have been developed as mimics of naturally-occurring high-density lipoproteins (HDL). Innaxon's Actives in Lipodisq™ which are biosynthetic, detergent-free, water-soluble novel lipid/polymer nanoparticles with stable encapsulated active ingredients, that retain the biological activity with enhanced bioavailability. |
 Download |
|
|
|