Cookie Policy: This site uses cookies to improve your experience. You can find out more about our use of cookies in our Privacy Policy. By continuing to browse this site you agree to our use of cookies.
Proteins with Enhanced Activity & Stability
AdipoGen Life Sciences specializes in the development of recombinant proteins that show enhanced activity and stability, produced in mammalian cells. Using proprietary in-house technologies together with established and published technologies, we engineer, develop and produce an innovative panel of recombinant proteins for different research fields including Cell Therapy Research.
The development of recombinant proteins is performed using different production expression systems, including the mammalian cell lines CHO and HEK 293. The mammalian cell lines allow AdipoGen Life Sciences to produce exogenous recombinant proteins secreted into the cell medium with post-translational modifications and glycosylations closer to endogenous proteins compared to productions in E. coli. However, for some difficult-to-produce in mammalian cells proteins, we also use bacteria (E. coli) and/or insect cells as a host. AdipoGen Life Sciences’ first goal is to provide high purity, low endotoxin and biologically active recombinant proteins, leveraging its in-house protein technologies.
Recombinant proteins are used throughout the life science academia and industry in a plethora of applications. They elicit their mechanism of action by activating signaling pathways through binding to specific cell-surface receptors. Their use ranges from cell culture to bioprocessing and advanced cell and gene therapy. A defined composition of the cell culture medium to guarantee batch-to-batch reproducibility has become inevitable. The development of animal-component-free recombinant proteins eliminates the risk of contamination that has been associated with animal and human serum-derived media.
AdipoGen Life Sciences' initiative to implement the production in serum-free (animal-component free) media conditions, copes with the need for its highly biologically active and stable proteins for these newly emerging markets and helps advance life sciences research. Ask for serum-free production lots!
Product Group Overview
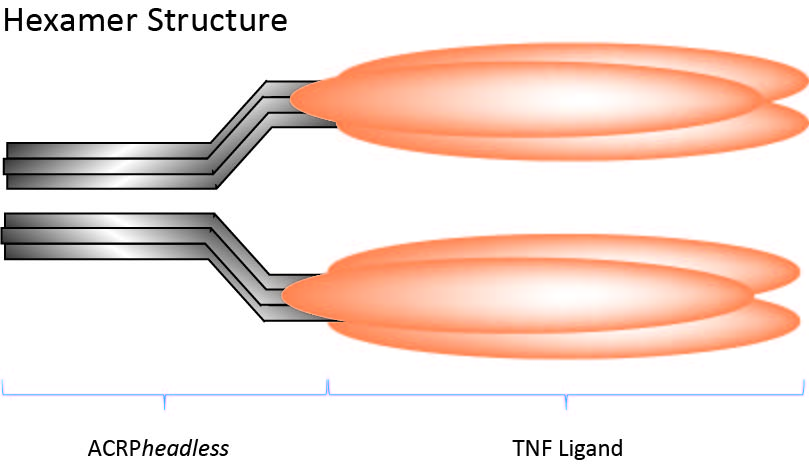
TNF Ligands Multimeric Proteins
For selected Products please see below or the full panel of Multimeric Proteins.
|
 |
| Product Name | PID | Host | Species | Activity |
| CD40L (human) (multimeric) (rec.) | AG-40B-0010 | CHO cells | Human |
Potent B cell and T cell expansion tool for Cell Therapy Application. Accelerates ex-vivo TIL expansion for Immunotherapy. |
| NEW CD40L (human) (multimeric) (rec.) (Certified Serum Grade) | AG-40B-0010CSG | CHO cells | Human |
Potent B cell and T cell expansion tool for Cell Therapy Application. Accelerates ex-vivo TIL expansion for Immunotherapy. |
| NEW B-Xpander™ CD40L (human) (rec.) (Animal Free) | AG-40B-0010AF | CHO cells | Human |
Produced using animal component-free medium in characterized and certified CHO cells. GMP-like production. Ex vivo B Cell Expansion for Cell Therapy. |
| CD40L (mouse) (multimeric) (rec.) | AG-40B-0020 | CHO cells | Human, Mouse | Potent B cell expansion tool. |
| FasL (human) (multimeric) (rec.) | AG-40B-0130 | HEK 293 cells | Human, Mouse | Potent apoptosis Inducer in human Jurkat T cells. |
| TNF-α (human) (multimeric) (rec.) | AG-40B-0019 | HEK 293 cells | Human, Mouse | Activates human and mouse TNF-R1 and TNF-R2. |
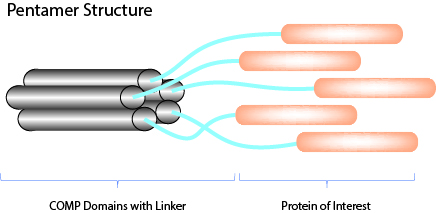
COMP-Fusion Proteins - Improved Avidity & Biological Activity
|
 |
| Product Name | PID | Host | Species |
| COMP (rat):Angiopoietin-1 (human) (rec.) | AG-40B-0147 | CHO cells | Human |
| VISTA (mouse):COMP (mouse) (rec.) (His) | AG-40B-0181 | HEK 293 cells | Mouse |
| VISTA (human):COMP (mouse) (rec.) (His) | AG-40B-0183 | HEK 293 cells | Human |
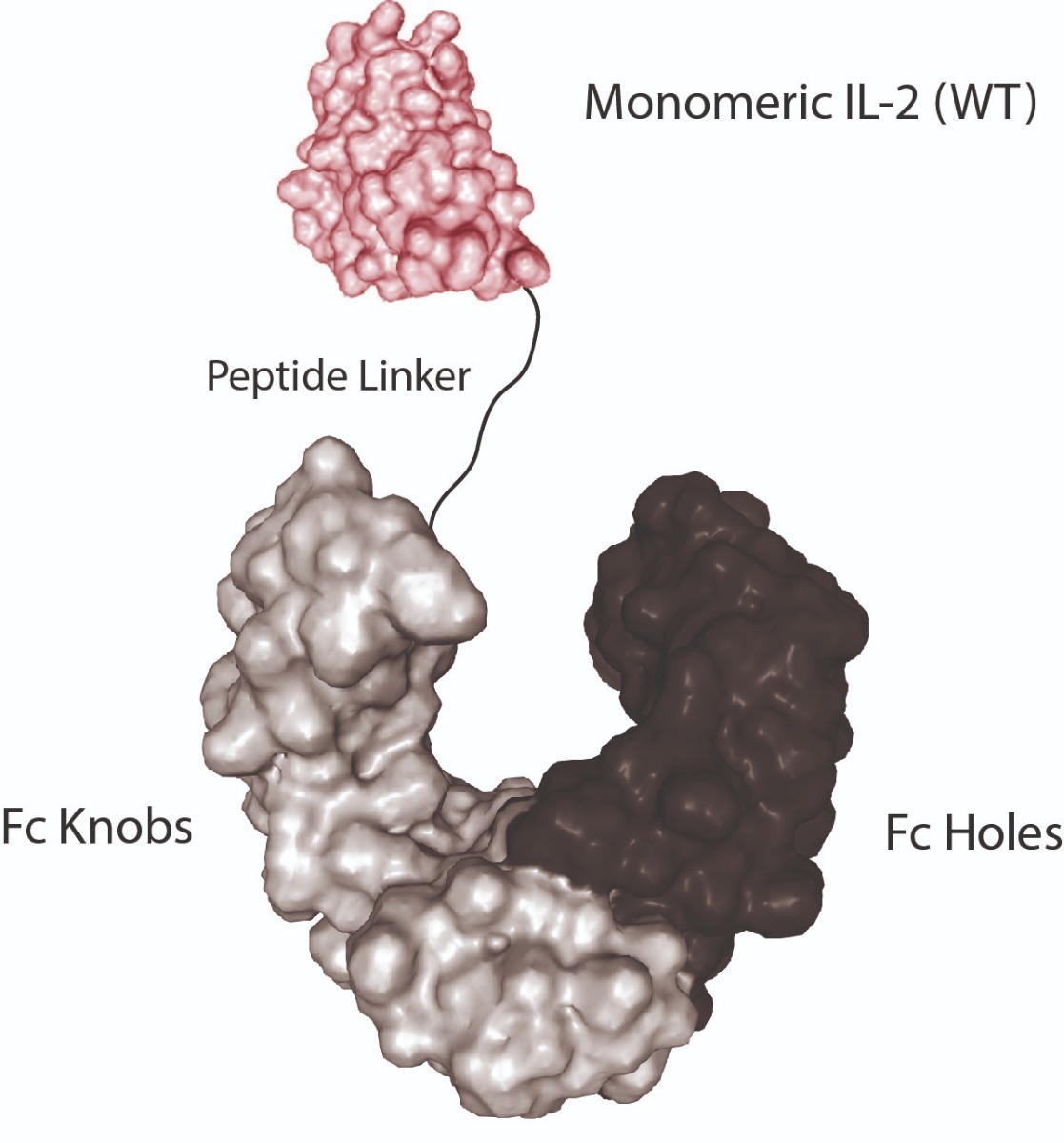
Monomeric Proteins fused to Fc (KIH-Technology): See www.invivokines.com for More Information!
Knobs-into-Holes (KIH) Technology:
Lit: J.B. Ridgway, et al.; Protein Eng. 9, 617 (1996)
|
 |
|
|
 |
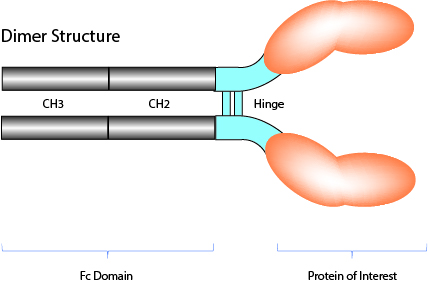
Fc-Fusion Proteins (wild-type or non-lytic)
|
 |
| Product Name | PID | Host | Species |
| LAG-3 (human):Fc (human) (rec.) | AG-40B-0031 | CHO cells | Human, Monkey, Mouse |
| LAG-3 (mouse):Fc (mouse) (rec.) | AG-40B-0039 | CHO cells | Human, Mouse |
| CTLA-4 (human):Fc (human) (rec.) (non-lytic) | CHI-HF-220A4 | CHO cells | Human |
| CTLA-4 (mouse):Fc (mouse) (rec.) (non-lytic) | CHI-MF-120A4 | NS1 cells | Mouse |
| IL-21 (mouse):Fc (mouse) (rec.) (non-lytic) | CHI-MF-12021 | CHO cells | Mouse |
| IL-35 (human):Fc (human) (rec.) | CHI-HF-21035 | CHO cells | Human |
| IL-35 (mouse):Fc (human) (rec.) | CHI-MF-11135 | CHO cells | Mouse |
| Jagged-1 (mouse):Fc (human) (rec.) | AG-40A-0157T | HEK 293 cells | Mouse |
| NEW VSTM5 (human):Fc (human) (rec.) (non-lytic) | AG-40B-0237 | HEK 293 cells | Human |
| NEW VSTM5 (mouse):Fc (mouse) (rec.) (non-lytic) | AG-40B-0239 | CHO cells | Mouse |
| Browse More: | |||
| All AdipoGen Life Sciences Fc Fusion Proteins | |||
| All Chimerigen Fc Fusion Proteins | |||
| All Chimerigen Non-Lytic Fc Fusion Proteins | |||
| All Ancell Fc Fusion Proteins |
Proteins with Site-directed Mutations
Site-directed mutagenesis is a valuable tool to modify genes and study the structural and functional properties of a protein, based on the structure, function, catalytic mechanism and catalytic residues of enzymes and therefore of high importance for protein engineering. The mutation may be a single base change (point mutation), multiple base changes (many mutations), deletion or insertion.
| Product Name | PID | Species | Protein Improvements |
| DLL4 (human):Fc (human) (rec.) (highly active mutant) | AG-40B-0176 | Human, Mouse | Several proprietary mutations result in a >20-fold increase in affinity relative to WT DLL4. |
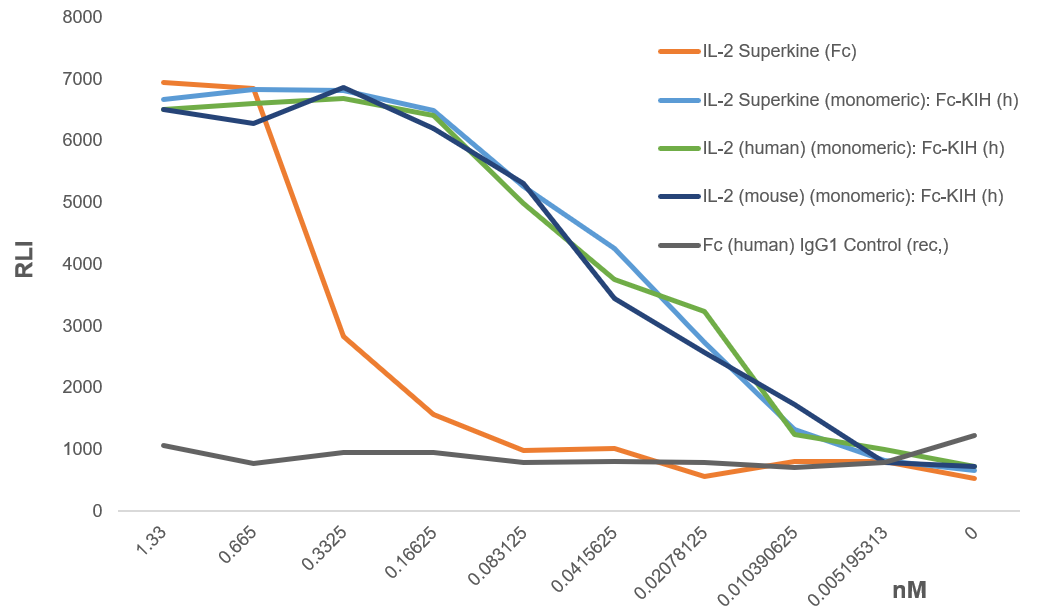
| IL-2 Superkine (Fc) | AG-40B-0111 | Human, Mouse | Mutations L80F / R81D / L85V / I86V / I92F are located in the molecule's core which acts to stabilize the structure and give it a receptor-binding conformation mimicking native IL-2 bound to CD25. These mutations effectively eliminate the functional requirement of IL-2 for CD25 expression and elicit the proliferation of T cells. |
| IL-2 Superkine (Fc) (H9T) | AG-40B-0219 | Human, Mouse | A new version of IL-2 Superkine with an additional mutation Q126T reduces the binding of IL-2Rγ and promotes the expansion of CD8+ T cells without driving terminal differentiation. |
| IL-15 (mutant) (human):Fc (human) (rec.) | CHI-HF-21015M | Human | Mutations at Q149D and Q156D make this protein specifically bind to the IL-15R, competitively inhibiting IL-15-triggered cell proliferation, promoting transplant tolerance and does not activate the STAT-signaling pathway. |
| IL-33 (oxidation resistant) (human) (rec.) (untagged) | AG-40B-0160 | Human, Mouse | Amino acids 208 and 232 are mutated from cysteines to serines to protect IL-33 from oxidation. |
| TGFβ1 (mutant) (human):Fc (human) (rec.) | CHI-HF-210TGFBM | Human, Mouse | Amino acids 33, 223, and 225 were mutated from cysteines into serines. This specific mutant protein containing the LAP+TGF-β1 domains allows the protein to be active. |
Selected Other Specialty Proteins
AdipoGen Life Sciences' R&D department has established in-house protocols for certain specialty proteins. These proteins have a special structure, multimerization or no tags. Only due to these special production protocols do these proteins elicit their biological activity. A few examples are listed below. A complete list of Untagged Proteins is available through the link.
| Product Name | PID | Host | Species |
| BAFF, Soluble (human) (60-mer) (rec.) (highly active) | AG-40B-0112 | E. coli | Human, Mouse |
| NEW CD152 [CTLA-4] (human) (rec.) (untagged) | AG-40B-0232 | HEK 293 cells | Human |
| Irisin (rec.) (untagged) (E. coli) | AG-40B-0103 | E. coli | Human, Mouse |
| Progranulin (human) (rec.) (untagged) | AG-40A-0188Y | HEK 293 cells | Human |
|
More Information |
|
Downloadable Flyer |
|
Proteins with Enhanced Activity & Stability - Mini-Catalog AdipoGen Life Sciences specializes in the development of recombinant proteins that show enhanced activity and long half-life, produced in mammalian cells. Using proprietary in-house technologies together with established and published technologies, we engineer, develop and produce an innovative panel of recombinant proteins for different research fields including Cell Therapy Research. Includes: • TNF Ligands Multimeric Proteins• Monomeric Interleukins (KIH-Technology) • COMP-Proteins • Fc-Fusion Proteins (wild-type or non-lytic) • Proteins with Site-directed Mutations • Proteins for Improving ex vivo T Cell Expansion and for Preclinical Research
|

